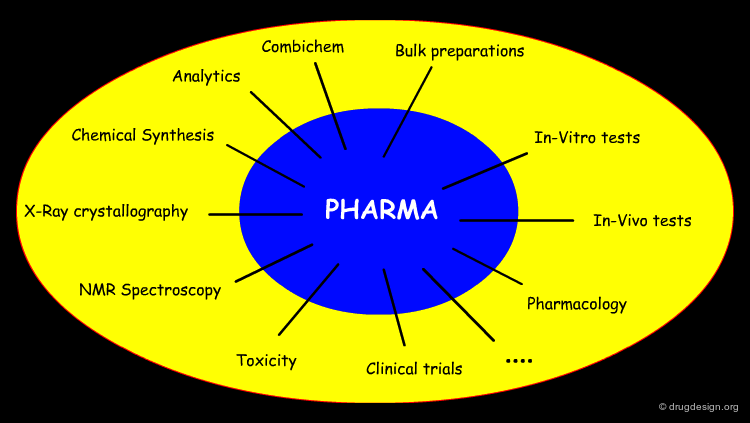
Introduction to Drug Discovery¶
Info
This is an introduction to drug discovery. After a brief history of the development of medicine from ancient civilizations to today, the following topics are discussed in this chapter: the revolutions that changed drug discovery, in-silico technologies, the drug discovery process, the rational drug design strategies, the major players in drug research and the role of chemistry in drug discovery. The chapter ends with an outline of the present and future aspects of drug discovery.
Number of Pages: 183 (±4 hours read)
Last Modified: July, 2011
Prerequisites: None
From the Origin of Medicines to Today¶
Attempts to Cure Diseases in Antiquity¶
Since antiquity people have tried to cure diseases by eating or applying substances they found in their natural environment. These attempts were based on intuition or on empirical observations.
Media
Medicines Used in Ancient Civilizations¶
The oldest records of medicinal preparations were made from plants, animals, or minerals by the early Chinese, Hindu, and Mediterranean civilizations.
5th-4th BC Centuries: The Greek Period¶
Hippocrates is probably the first physician to reject superstitions with causing illness. His therapeutic approach was relying on the healing power of nature. He revolutionized medicine in ancient Greece, establishing it as a discipline distinct from other fields traditionally associated with philosophy and religion. Hippocrates is widely considered to be the father of medicine.
Media
2nd Century: The Roman Period¶
In the ancient Rome Galien conceived a number of drugs; at this time they were searching for universal drugs which were conceived as a mixture of many drugs; some of them could contain more than 100 components. He considered that the human body is formed of the four elements, earth, water, air and fire which shape the outside world, and which contain four humours, blood, choler, phlegm and melancholy; the disease results from a loss of balance between all these elements. He perpetuated Hippocratic medicine and used opium for the relief of pain.
Media
13th Century: The Arab School¶
In the 13th century, with Avicenna, Ibn-al-Baytar and many others, the Arab school contributed important achievements. Ibn-al-Baytar described more than 2000 medicines in his Kitab al-Jami fi al-Adwiya al-Mufrada translated into latin as Corpus Simplicium Medicamentorum.
13th Century: First Apothecary Shops¶
It is only in the 13th century that the first apothecary shops appeared; they were preparing and selling herbal medical preparations. People relied on apothecary shops for treatments for every type of ailment, from tetanus to tumors, from headaches to hair loss. The first apothecary shops were founded during the Middle Ages in Baghdad (8th century).
Media
16th Century: Pharmaceutical Science¶
Pharmaceutical science emerged as a science in the 16th century. The Swiss physician-chemist Paracelsus advocated to abandon the illusive quest of gold by alchemy and to use chemistry for the preparation of medicines. The first pharmacopoeia appeared in Germany in 1542 with an inventory of drugs with instructions on their use and preparation.
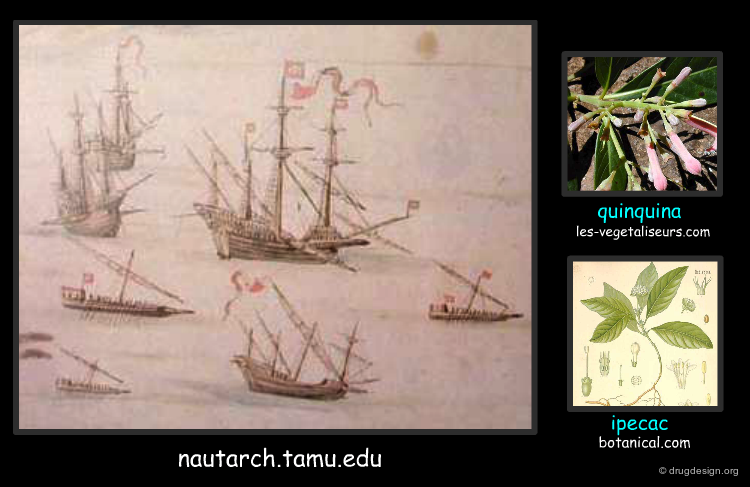
16th Century: Maritime Routes to India and America¶
Further to the discovery of new maritime routes to India and America new substances such as tea, coffee, cacao, spices and other medicines as quinquina and ipecac were introduced in Europe in the 16th and the 17th centuries; they opened a new era in drug therapy.
18th Century: First Vaccine¶
In 1796 the English surgeon Edward Jenner conceived the concept of vaccination by observing that dairymaids seemed to have immunity to smallpox. He used the cowpox virus to test the idea and the trial proved to be successful: the principle of vaccination was born. The idea was exploited ninety years later by Louis Pasteur for the development of rabies vaccine; these were a major breakthrough in the fight against these new types of diseases.
Media
19th Century: Drug Administration¶
It was not always clearly understood how medications must be delivered in order to be effective. Apothecaries made pills that were designed to be swallowed, pass through the gastrointestinal tract, be retrieved from the stool, and used again. However, it was found that not all drugs could be absorbed into the body from the gastrointestinal tract. Other routes of delivery were searched where drugs could be injected directly into the bloodstream; this led to the design in 1853 by the French surgeon Charles Gabriel Pravaz of the hollow hypodermic needle that was first tested on dogs. These discoveries were of great significance and represent the first steps in drug dosage and administration.
Media
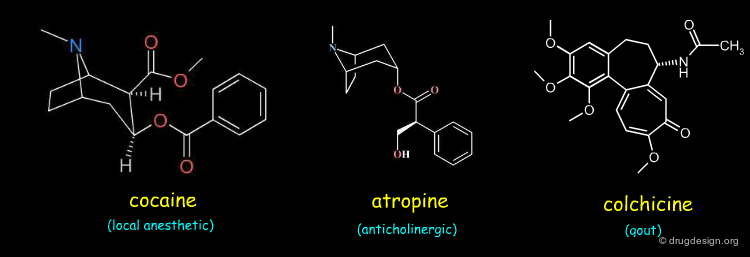
19th Century: Isolation of Compounds¶
The 19th century was the time of characterization of natural products by breaking them down into simpler components. It was also the "organic synthesis" time aiming to transform and synthesize organic compounds. Synthetic chemistry enabled the discovery of many therapeutic agents such as chloral, chloroform, aspirin, salicylates and quinine.
19th Century: Relief of Pain in Surgical Operations¶
Pain relief has been a priority for surgeons, and the discovery of ether in 1842 and chloroform in 1847 as anesthetics enabled them to take pride in the speed they could do a surgical operation. This also helped to reduce the fear and anxiety of the patients.
Media
19th Century: New Classes of Drugs¶
Phenol started to be used as an anti-infective agent in the prevention of infectious diseases and chloral hydrate was introduced and became the first synthetic sedative-hypnotic drug. Nitroglycerin was found as a relaxant agent (it was later found that this class of organic nitrates are also useful in the treatment of heart problems). A number of new agents were introduced during this period as for example acetanilide (analgesic-antipyretic), the artificial sweetener saccharin and benzocaine (the first of many local anesthetics).
20th Century: Pioneer Work of Paul Ehrlich¶
Following an outstanding pioneer approach Paul Ehrlich developed in 1910 Arsphenamine for the treatment of syphilis. This was the first drug synthesized to kill a specific invading microorganism. Ehrlich was the first who synthesized and tested a large number of compounds to find the most effective molecule. This screening approach remains one of the most important methods used by the pharmaceutical industry to discover new drugs.
Media
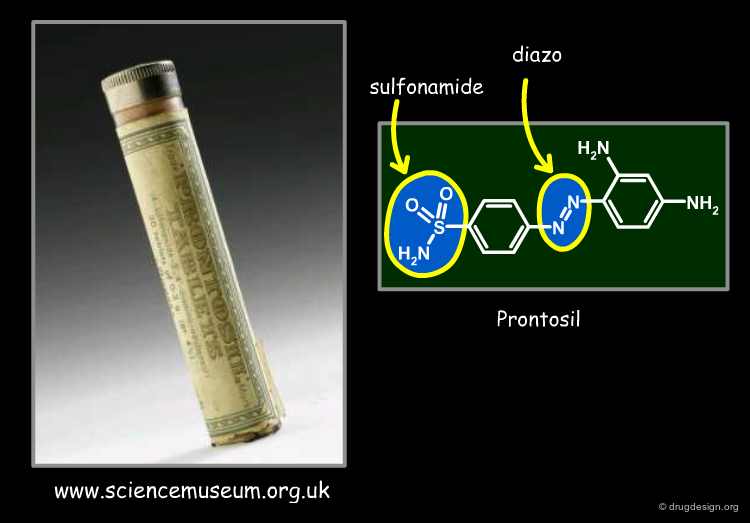
20th Century: Sulfonamide Antibacterial Dyes¶
In the 1930's, it was found that diazo dyes containing sulfonamide groups were effective in treating streptococcal infections in mice. The first one was developed as antibacterial agent and is known as Prontosil. Many sulfonamide analogs were then synthesized and tested for their biological activities.
Media
20th Century: Recognition of Vitamins¶
Vitamins are necessary for body metabolism and must be provided from the diet. Most of the vitamin deficiency disorders were proven in the late 19th and early 20th centuries. An example proving their importance is the dramatic story of the Japanese sailors who suffered from the terrible beriberi disease. Japanese sailors main diet consisted of polished white rice, which is very poor in thiamine (vitamin B1). The preliminary polishing treatment of the white rice removed the important thiamine, a molecule required for glucose metabolism and for construction of neuron membranes, among other functions.
Media
20th Century: The Antibiotic Era¶
Penicillin was discovered by Alexander Fleming in 1928 but he was unable to prepare enough material to carry out human trials. The discovery remained a laboratory curiosity. Ten years later Florey and Chain at Oxford University purified penicillin in sufficient quantity to collect new evidence on the remarkable power of the molecule and to prove its value as a drug. They imagined the setup of a series of small factories in UK for the production of the molecule but finally in the early 1940s, they sought help in America.
20th Century: Pfizer Management Decision¶
Florey played a crucial role in convincing the US pharmaceutical companies to undertake the large-scale production of penicillin. The project was very risky; however Pfizer's management took the leap and voted the investment of millions of dollars to buy the equipment and facilities needed for the mass production.
20th Century: Large Scale Production of Penicillin¶
Pfizer succeeded to develop a process for producing penicillin based on deep tank fermentation techniques, a process which was shared with 19 other US pharmaceutical companies. Looking for massive quantities to support the war effort, the U.S. government financed the project and soon Pfizer was producing five times more penicillin than originally anticipated. The drug started to be used by the Allied forces in Normandy in 1944 and for the rest of the war; it contributed saving countless lives.
Media
20th Century: Antibacterial Agents Isolated from Plants¶
An important era of antibiotic discovery took place in the mid 20th century; a great number of antibacterial agents were isolated from plants as for example streptomycin, choramphenicol and tetracyclines. In particular the American biochemist Selman Abraham Waksman (1888-1973) contributed to the discovery of Streptomycin and over twenty other antibiotics - a word which he coined.
20th Century: Discovery of Adrenal Cortex Hormones¶
In the 1930's Edward Calvin Kendall discovered the adrenal cortex hormones and was awarded the 1950 Nobel Prize for Physiology and Medicine. He isolated the adrenal cortex hormones and demonstrated their structures, and their functions. Lew Sarett designed at Merck & Co. a practical synthesis of Cortisone and the molecule was first produced commercially in 1949.
20th Century: NSAIDs¶
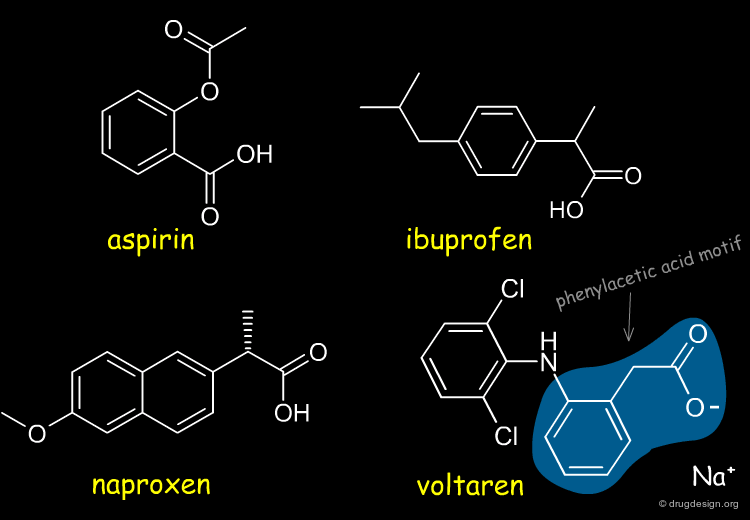
Non-steroidal anti-inflammatory drugs (NSAIDs) are analgesic drugs that were discovered in the 1960s and represent a major breakthrough in drug discovery. This is a new class of drugs that relief pain and reduce inflammation. Aspirin, ibuprofen, naproxen and voltaren are examples of members of this class. Most of them share the common phenyl-acetic-acid motif.
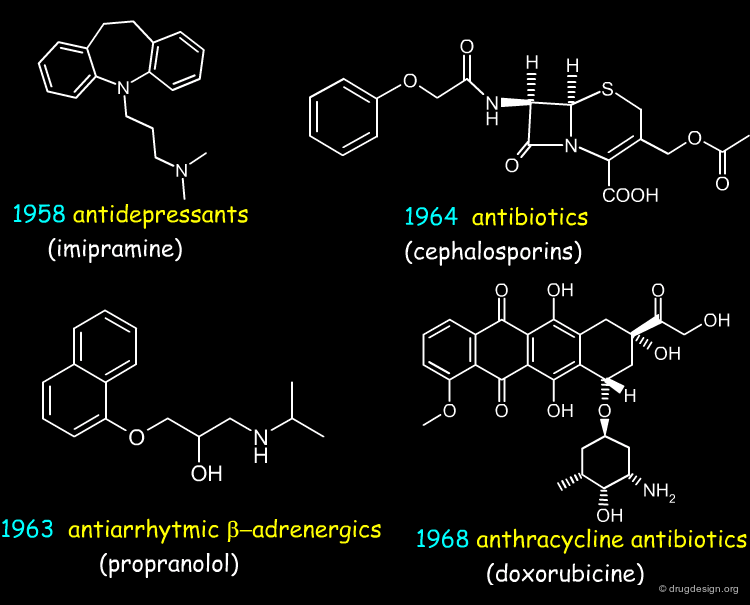
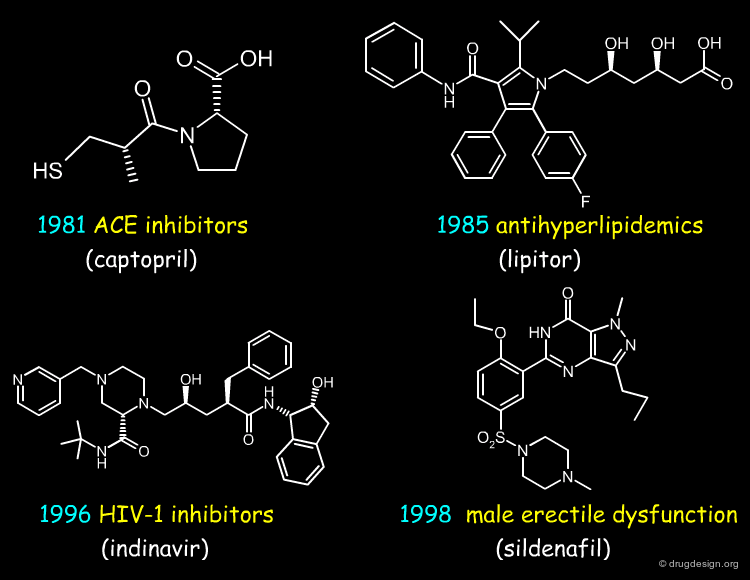
20th Century: Key Transition Discoveries¶
From the second half of the 20th century appeared many other classes of drugs. The list presented below (click the buttons) indicates some of them, with a typical example for each. The list is far from being exhaustive, it just gives an idea of the new dimension acquired by drug discovery in the pharmaceutical industry in the course of that period.
20th Century: Some Drug Discovery Projects¶
The following list gives some particular projects in which substantial effort was spent in R&D in the 20th Century to find new therapeutic agents. The examples indicated in red are presented with some detail in the following pages.
Example-1 of Project: Factor Xa¶
Factor Xa plays a critical role in the coagulation cascade; its inhibition is expected to lead to antithrombotic agents. New drugs were searched by targeting inhibition of this protein and the design strategy was done based on its 3D structure. This led to the discovery of a new and potent Factor Xa inhibitors such as the molecule shown below.
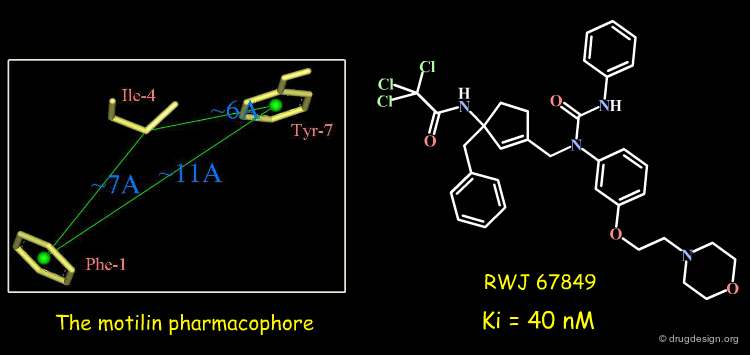
Example-2 of Project: Motilin Antagonists¶
Motilin is a 22 amino acid peptide secreted in the small intestine that stimulates the contraction of smooth muscle in the gastrointestinal tract. Many motility disorders are currently treated through diet and stress management; an orally active small molecule motilin antagonist was searched to be used for regulating gastrointestinal motility. The molecule described below was found using a 3D pharmacophore model of the motilin hormone.
Example-3 of Project: COX-2 Inhibitors¶
In the mid 1990's pharmaceutical companies searched for a new class of anti-inflammatory agents termed COX-2 inhibitors expected to have advantages over conventional non-selective COX inhibitors (NSAIDs) since the gastrointestinal effects such as ulceration, and internal bleeds are mediated by COX-1. In 1999, the U.S. Food and Drug Administration approved Vioxx that became a blockbuster with annual sales over $ 2 billions. In 2004 Vioxx was withdrawn from the market due to increased risk of heart attack and stroke.
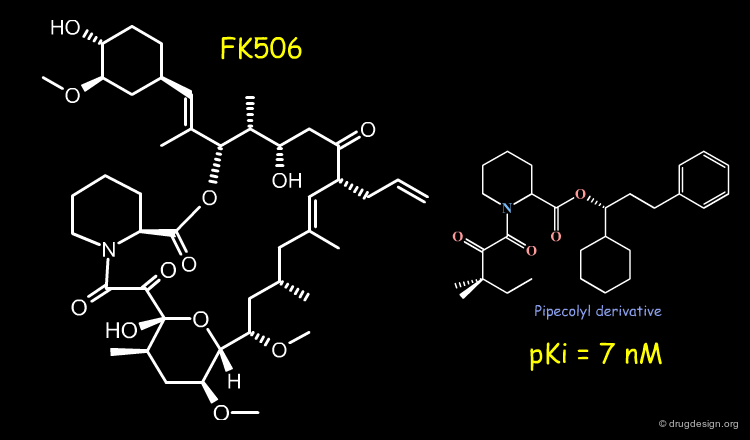
Example-4 of Project: Rotamase Inhibitors¶
FK506 is a microbial immunosuppressant product structurally related to cyclosporin which inhibits rotamase activity and disrupts essential T-cell activation events. Structure-based and pharmacophore-based design techniques were used to identify the essential binding groups of FK506 and to conceive high-affinity rotamase inhibitors with reduced molecular complexity. An example of inhibitor is shown below.
20th Century: From Apothecaries to Factories¶
Major pharmaceutical companies were founded in the late 19th and early 20th centuries; they gradually superseded the traditional compounding of medications by individuals. For example, in the Upper Rhine Valley near Basel, Switzerland new factories produced dyestuffs with antiseptic properties. Some of the leading pharmaceutical companies, including Hoffman-La-Roche, Sandoz, Ciba-Geigy and, later, Novartis were founded in this region.
Revolutions that Changed Drug Discovery¶
Major Achievements¶
What changes gave a new dimension to today's drug discovery ? We present most important of them in the following pages.
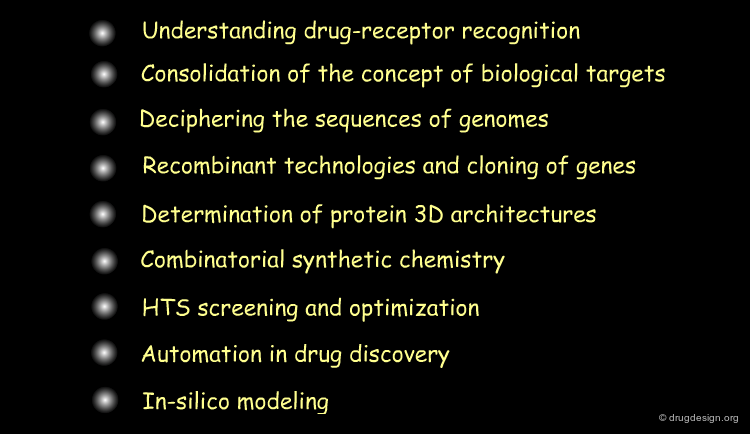
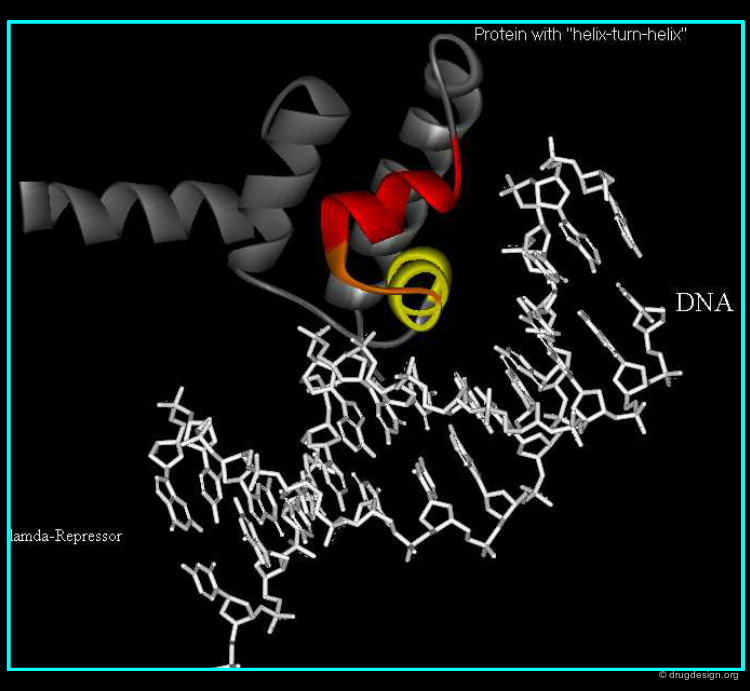
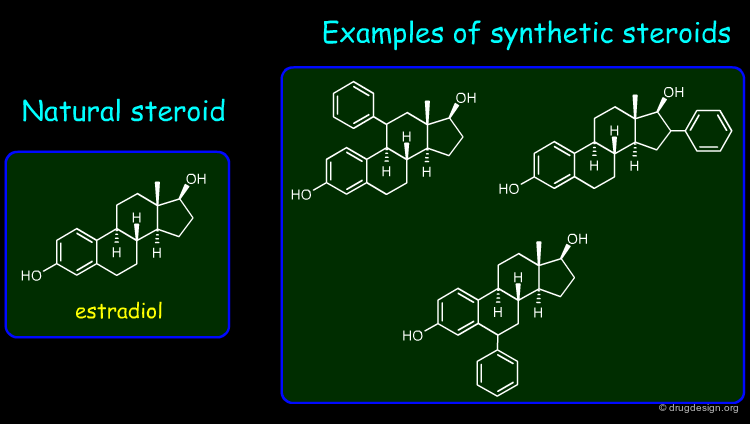
Understanding Drug-Receptor Recognition¶
A concept of great importance reveals why and how the interaction of a drug with its receptor depends on the exact 3D features of the two molecules. This principle proved to be of central importance in drug discovery for understanding the origin of a disease at the molecular level and a key for developing new drugs.
Consolidation of Concept of Biological Targets¶
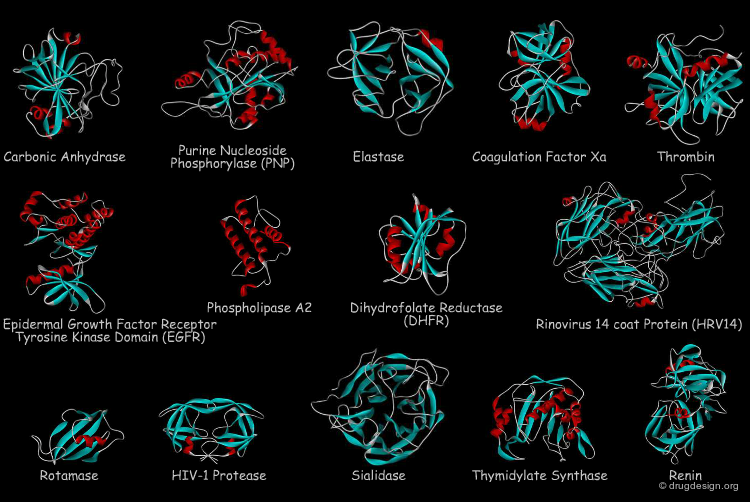
Recombinant technologies permitted to widen drug discovery in strengthening the concept of biological targets. These are proteins such as enzymes, ion channels, and receptors whose activity are attempted to be modified by an external molecule in order to create new therapeutic agents. About 600 proteins are now used as targets in the pharmaceutical industry; they belong to the gene families indicated in the pie chart below.
Recombinant Technologies and Cloning of Genes¶
In the 1990's recombinant technology created the biotechnology industry. It enabled the X-ray determination of key protein structures and began to play a concrete therapeutic role in enabling structure-based design to be exploited in drug discovery. The principle is to insert the DNA from any source into bacteria and to express the corresponding gene in these cells. Somatostatin was the first human protein made using this technology.
Media
Deciphering the Sequences of Genomes¶
The Human Genome Project opened up many novel strategies in drug discovery enabling the identification of new areas for therapeutic intervention. The primary goal of this project was to determine the sequence of chemical base pairs which make up DNA, to identify disease-relevant targets that could be exploited to prevent and cure many diseases. Today, the genomes of many organisms have been deciphered and help to identify abnormalities and derive specific drugs that address them.
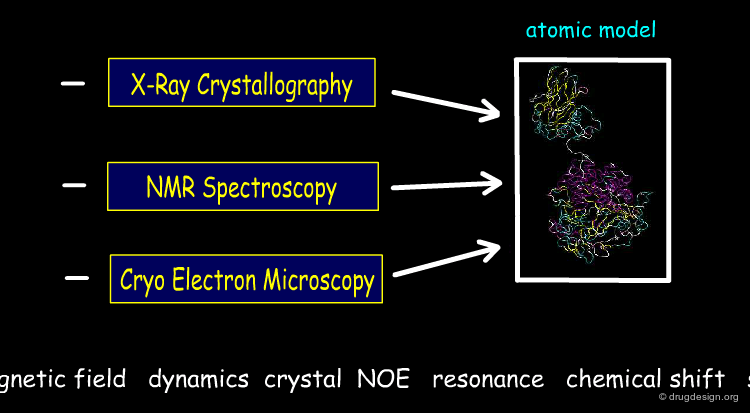
Determination of Proteins 3D Architectures¶
The 3D architectures of proteins give an important means to understand biological structure and function; in particular they provide insight into the subtle interaction between a drug and its target receptor, an information of utmost importance in drug discovery. Today the 3D structures of over 60,000 proteins, nucleic acids and other biological molecules have been determined by X-ray crystallography and NMR spectroscopy.
Automated Methods in Synthetic Chemistry¶
Automated methods in synthetic chemistry for reaction design, information management and compound analysis opened up a new era in synthetic chemistry. Whereas talented synthetic medicinal chemists could synthesize 3-5 compounds a week by classical methods, they can now prepare an order of magnitude more compounds using automated methods. Automated methods generate data of high informational content for drug discovery and contribute to accelerate the discovery process.
HTS Screening¶
High-throughput screening (HTS) has become one of the most important methods for lead identification in drug discovery. It uses automation to run a screen of an assay against a library of compound candidates. Typical HTS screening libraries can contain from 100,000 to more than 2,000,000 compounds. Lead optimization can be speeded by using combinatorial chemistry technologies and testing the molecules by HTS technologies.
Automation in Drug Discovery¶
In all fields of drug discovery, computer-assisted automation of laboratories has accelerated many aspects of the discovery process. Dedicated machines perform a number of repetitive functions for specific tasks as for example in high throughput screening, genomics, chromatography, crystallization, mass spectroscopy, analytics, clinical screening, ADME and toxicology assays.
In-Silico Modeling becomes Mature¶
A major revolution took place in drug discovery with the development of in-silico software. This became possible with the advent of high performance workstations, computer graphics and X-ray protein determinations. In-silico molecular modeling has emerged as a major new tool in drug discovery. In the following section we present the different in-silico disciplines that made this possible.
In-Silico Technologies¶
The Explosion of In-Silico Technologies¶
Computers have triggered the emergence of in-silico technologies that constitute the essence of modern drug discovery. A description of some of them is presented in the following pages.
Encoding Molecules: 2D and 3D Databases¶
2D and 3D structures are of central importance in drug discovery. The molecular architectures can be analyzed, searched, visualized and compared with other structures; all these are standard operations in everyday drug discovery research. As the practice of manipulating molecular structures with computers has matured, specific methods and standards for encoding 2D and 3D structures were fixed and adopted by the scientific community.
Development of Molecule Encoding¶
Computer-readable representations of 2D chemical structures started to be introduced in the mid 1960's and paved the way to 2D substructure searches. It took about ten more years to incorporate the 3D descriptors that enabled 3D substructure searches. This was one of the first type of application that opened up an entire new era in the development of modern computer-aided tools in drug discovery.
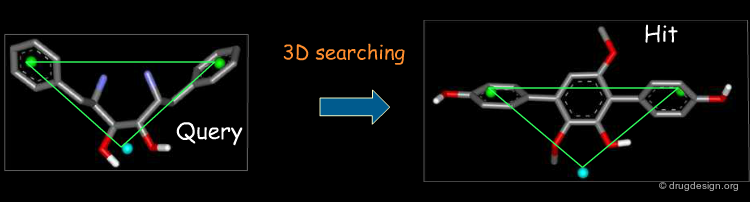
3D Searching is not Trivial¶
3D searching is difficult because molecules can assume different conformations, but only certain conformations are likely associated with bioactivity.
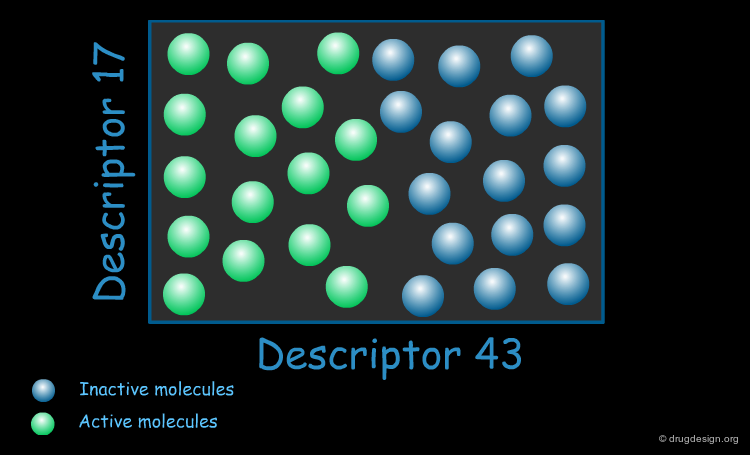
Encoding Molecular Properties¶
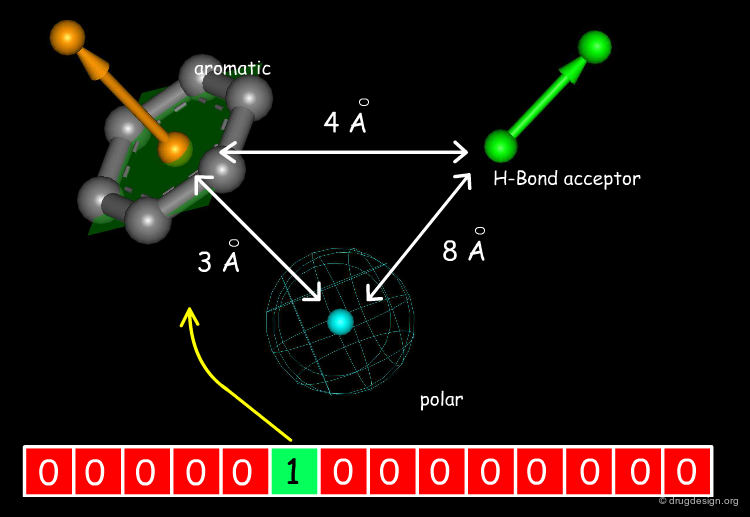
Other molecular properties can be encoded. For example the structural approach to substructure searching is based on bitmaps (also referred to as "fingerprints") and have bits set according to the presence or absence of certain structural features in the molecule. Each bit represents a structural descriptor. The premise of the similarity comparison is that similar structures have similar fingerprints. An example of a pharmacophore encoding is shown below.
Development of Models for Data Analysis¶
The accumulation of data collected on molecules triggered the need of using it to generate new and useful information for drug discovery. This started to be exploited in the 1970's by using molecule visualization and statistical analysis methods. Examples of such applications are presented in the following pages.
Molecular Modeling¶
Molecular modeling technologies started to emerge in the mid 1980's and gave great impetus to rationalizing drug discovery. It gave a new dimension to drug design by enabling the intelligent exploitation of 3D molecular properties.
Development of Computer Graphics¶
Computer graphics greatly helped in understanding complex processes such as molecular interactions and structural complementarity through the precise visualization of 2D and 3D molecular structures. It also helped chemists to calibrate their chemical intuition and visualize relationships that may be difficult to compute. All these represent the very basis of molecular analyses in drug discovery.
Development of Models for Data Visualization¶
Data modeling and visualization have been introduced in a number of aspects of drug discovery. For example they provided useful models in the development of structure-based technologies for complex systems to exploit this knowledge for designing new molecules.
In-Silico Molecular Mechanics¶
Molecular mechanics is an in silico technique developed in the 1980s to simulate the 3D behavior of molecules. This information is of utmost importance in drug discovery for comparing molecular structures in 3D and for studying the interactions of small molecules docked to macromolecules. The method is based on a set of empirical energy functions called a force field describing the total energy of the system.
In-Silico Molecular Dynamics¶
In structure-based design the knowledge of the molecular motions involved in the target protein are of key importance for enabling reliable analyses and design of molecules and the prediction of the properties of new compounds. Molecular dynamics is the most commonly used simulation technique for studying these motions. The first widely available program based on this technology was AMBER (introduced in 1981).
In-Silico Docking¶
As the practice of molecular modeling has matured, a method named in-silico docking has developed for predicting the optimal binding of interacting molecules and to estimate the energy of their complex. The method has become widely used in drug discovery to design small molecules that interact optimally with a target protein of known 3D structure. However, one should note the limited accuracy of docking calculations due to the complexity of the systems.
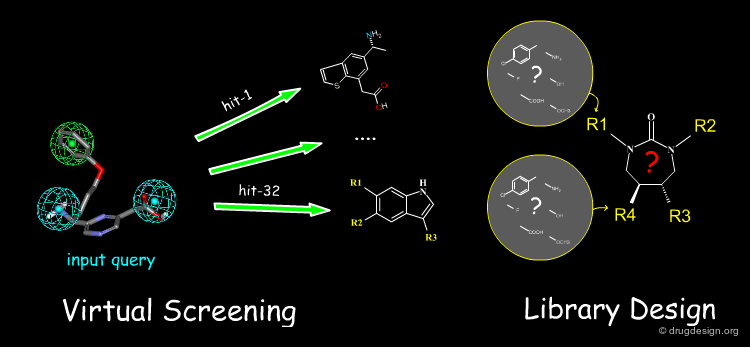
In-Silico Virtual Screening¶
Computerized databases started to be exploited in drug discovery in the early 1990s to find molecules in a database that contain structural elements as defined by a 2D or a 3D query or other properties. The technique is known as Virtual Screening. In drug discovery, in-silico virtual screening has been developed either in a ligand-based (2D or 3D similarity with the input query) or in a structure-based perspective (complementarity with the 3D structure of a receptor).
3D Searching and Bioisosterism¶
In drug discovery it is often useful to find potential bioactive molecules chemically unrelated to that of a reference active one (e.g. bioisosteres). 3D searching is a technique that is based on searching in a database of molecules for compounds that contain structural elements as defined by a 3D query. The potential bioactive molecules can be recognized, based on their 3D properties.
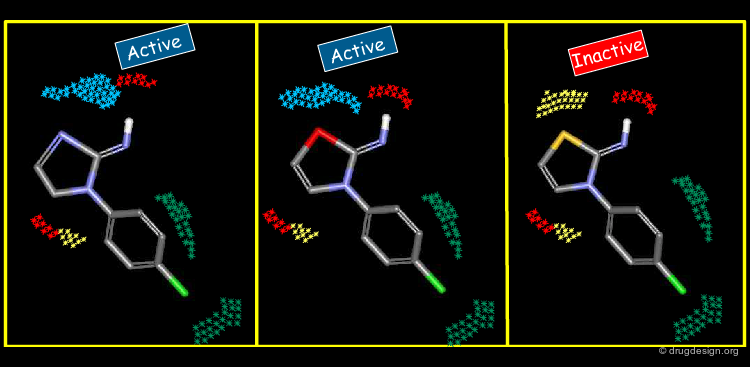
Pharmacophore Elucidation and Mapping¶
One of the methods employed in ligand-based drug research is to find new structural scaffolds by exploiting pharmacophore mapping. It is a computerized approach used in drug discovery to derive 3D pharmacophores based on the 3D alignment of the geometric and physicochemical features of known active reference compounds.
Structure-Activity Relationships - SAR¶
SAR analyses try to convert structure-activity observations into informative structure-activity relationships. They aim at maximizing the knowledge that can be extracted from raw data in molecular terms, exploiting this knowledge to identifying lead compounds for either additional modifications or further pre-clinical studies. This represents an important means to fully exploit the diverse knowledge accumulated in a project.
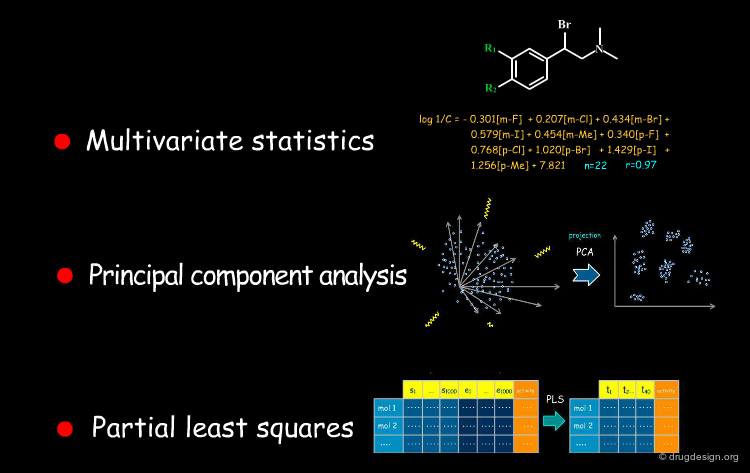
Chemometrics¶
As the practice of statistical analyses has matured, a new discipline emerged, called "chemometrics" which is of great utility in drug discovery. It consists of applying mathematical models and statistical methods to chemical data. Multivariate statistics, principal component analysis and partial least squares are examples of methods used in chemometric analyses.
Development of QSAR¶
Mathematical models that relate descriptors (properties) derived from the chemical structure have been developed for drug discovey to predict the in-vitro and in-vivo activities of a compound before it is synthesized. It greatly contributed to introducing the computational dimension in drug discovery. QSAR (Quantitative Structure-Activity Relationships) has been conceived by Corwin Hansch in the early 1960s.
3D-QSAR¶
25 years later, 3D-QSAR emerged as a follow-up of the initial QSAR approach. 3D-QSAR is a method generally used in drug discovery situations using statistical correlation analyses to compare the 3D molecular forces ("fields") produced in the vicinity of different compounds in order to find correlations between biological activities and fields. This method generally applies in drug discovery in situations where the structure of the receptor is not known. It started to be used in research projects in the early 1990's.
In-Silico Library Design¶
In drug discovery, when a synthetic scheme is available on paper a great number of diverse molecules can be prepared. Library design is an in-silico methodology that reduces the number of molecules which need to be made, without decreasing the diversity of the library produced. It is based on the assumption that structurally dissimilar molecules are likely to have different properties.
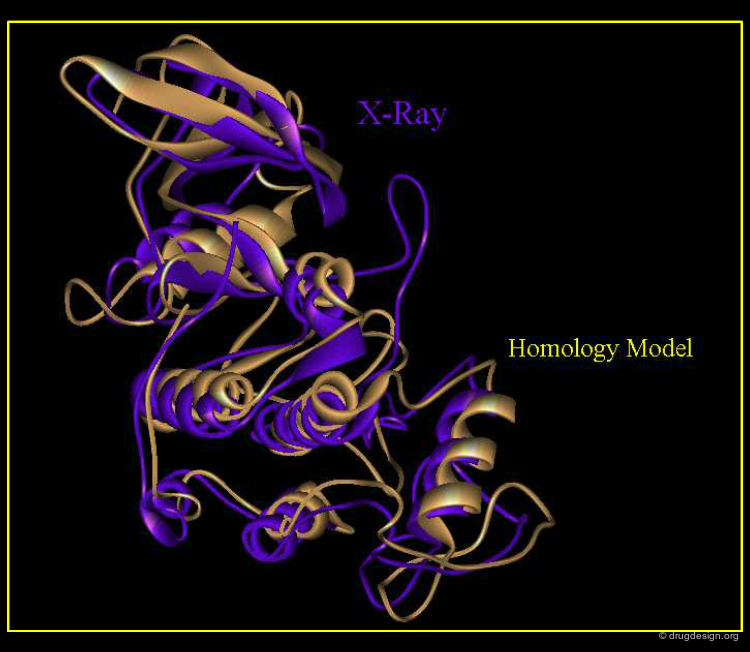
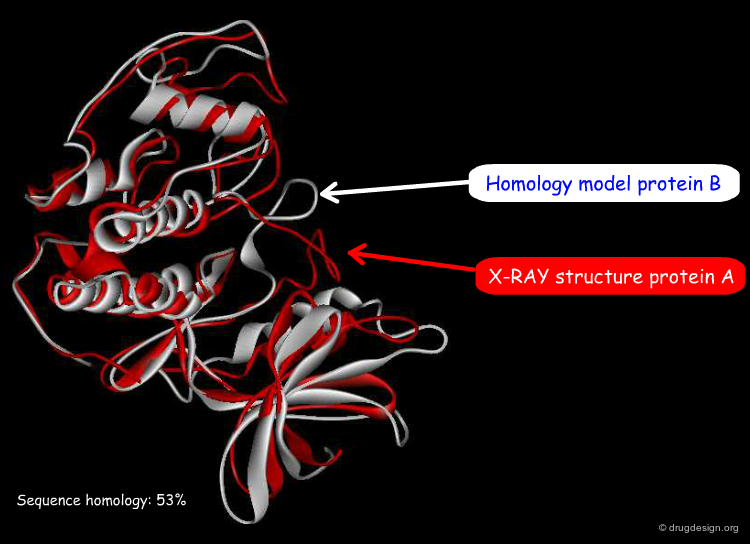
In-Silico Homology Modeling¶
For structure-based design applications in drug discovery, the 3D structure of the target protein needs to be available. In-silico homology modeling has been developed to generate 3D models of a protein of known sequence and unknown structure. The basis of homology modeling is the assumption that proteins with sufficient sequence homology are likely to fold similarly. The primary requirement is to detect sequence similarities with proteins of known X-ray structures. Sequence alignments are then used to produce structural models of the protein considered.
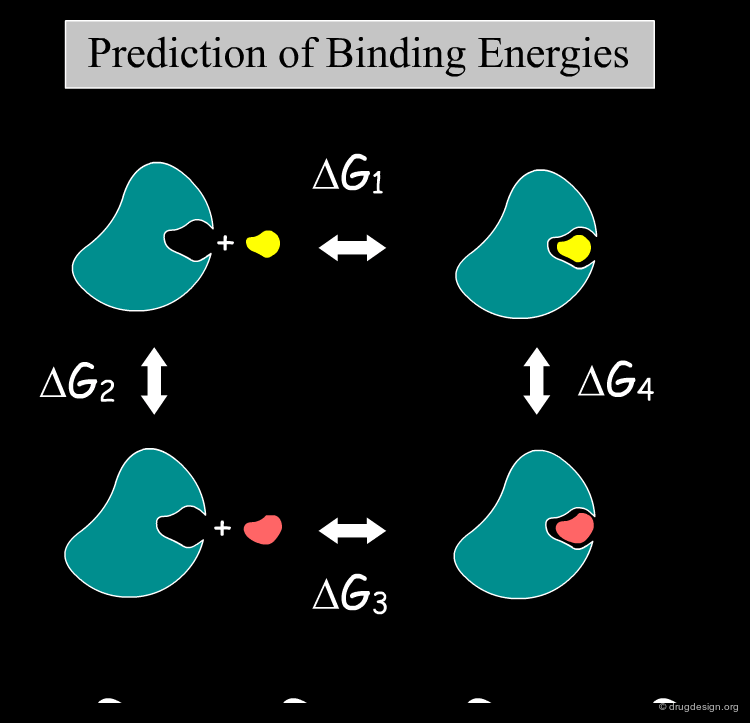
In-Silico Free Energy Simulations¶
Knowing the binding energy of a ligand to a protein is of utmost importance in drug discovery. Based on molecular dynamics, and the experimental binding free energy of a known inhibitor, it is possible to calculate the free energy of binding of other inhibitors with a method called "Free Energy Perturbation (FEP)". The method requires substantial computer time and is used by specialists in appropriate cases.
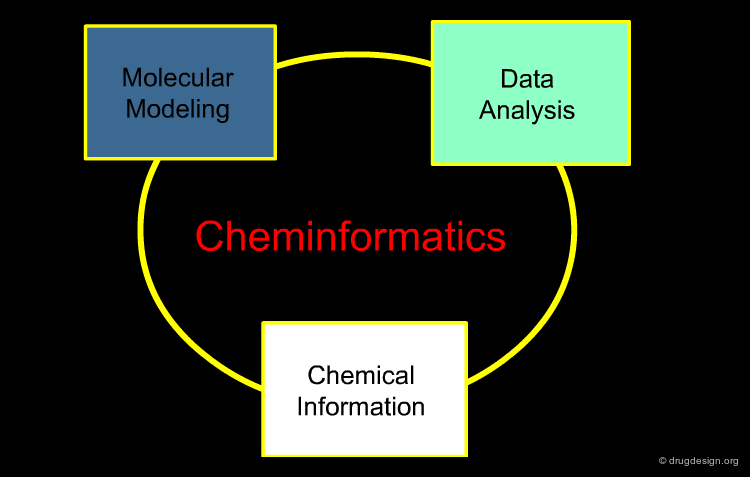
Cheminformatics¶
Cheminformatics has become a rapidly growing field that emerged in drug discovery in the late 1990s. It is an inter-disciplinary field involving chemistry, physics, mathematics, computer science and information technologies. It assembles molecular modeling, data analysis and chemical information within this new discipline.
Structural Bioinformatics¶
Structural Bioinformatics is a recent drug discovery interdisciplinary field that deals with the three dimensional structures of biomolecules. It attempts to model and discover the basic principles underlying biological machinery at the molecular level. It is based on the belief that 3D structural information of a biological system is the core to understanding its mechanism of action and function. Structural bioinformatics combines applications of physical and chemical principles with algorithms from computational science.
Reaction Searching¶
Reaction searching is the capability to input the structure of a starting material and/or the structure of a desired product and conduct a reaction query. In the past the Chemical Abstracts were used in drug discovery for searching reactions; but in recent years many specialized reaction chemistry databases were developed to assist the chemist in a search for reaction information. This information is of great importance for drug design applications.
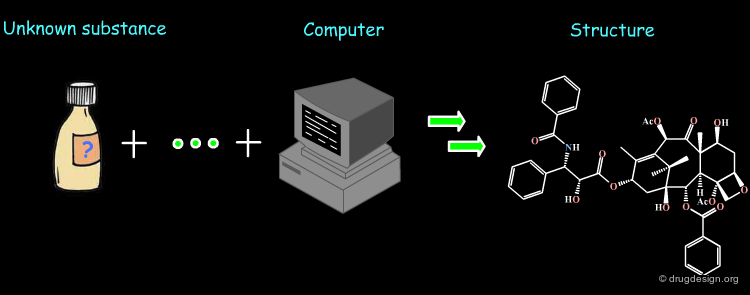
Computer-Assisted Structure Elucidation¶
With the advent of powerful computers, computer-aided structure elucidation methods using spectroscopic data, were developed. They bring useful contributions in drug discovery for helping in the determination of the structure of new compounds.
In-Silico Prediction of ADME/Tox Properties¶
To maximize the drug discovery effort and to cut the attrition rate in drug development, advanced software programs started to be developed aiming at creating in-silico models to be used to predict ADME/Tox (Absorption, Distribution, Metabolism, Excretion and Toxicity) properties of a candidate drug.
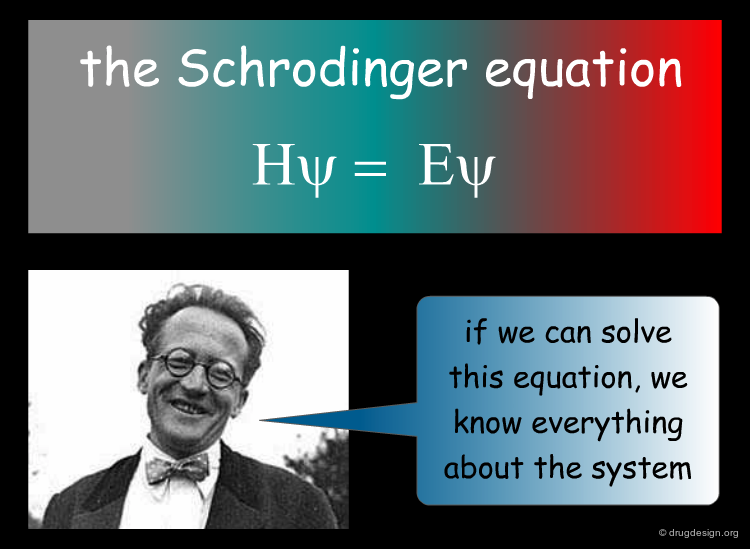
Quantum Chemical Calculations¶
As already mentioned, the binding energy of a ligand to a protein is of utmost importance in drug discovery applications. In theory, the best mathematical calculation of the energy of a molecular system is the Schrodinger equation. Unfortunately in current practice, the resolution of the equation is far beyond current state of the art in this field, but approximate quantum mechanical methods can contribute useful information about binding energies and reactivities.
The Drug Discovery Process¶
Outline of Drug Discovery¶
The drug discovery process involves the identification of a promising hit, the transformation of this hit into a lead and optimization of the lead according to multiple criteria presented later in this section. It is also during that phase that patent applications start to be filed. This R&D phase generally requires 4-5 years and may cost about $40 millions dollars to the pharmaceutical company.
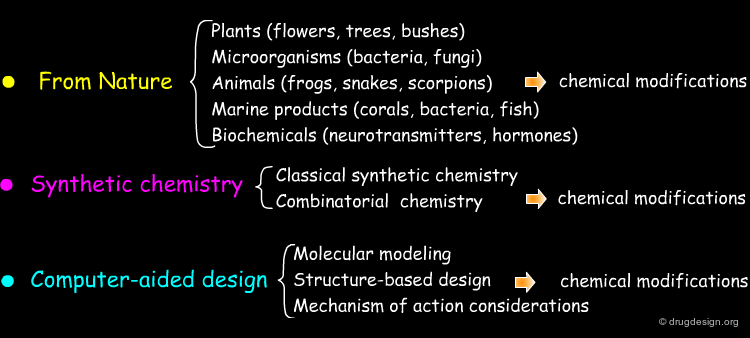
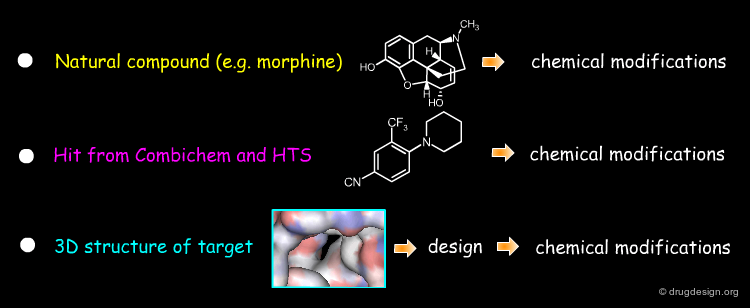
Starting Idea in a Project¶
The starting idea of a project can be very different from one to another project. Below are shown some examples of ideas that may be used to initiate a drug discovery project. The list is far from being exhaustive.
Importance of Assay Validation¶
Assay validation is an important issue in drug discovery. In vitro and animal assays, and even human biomarkers, can be misleading unless they have been demonstrated to be pertinent to the clinical indication. Recent advances with humanized cells and tissues improve relevancy of the in vitro and in vivo assays, but correlation with therapeutic efficacy is still necessary.
The Drug Discovery Process¶
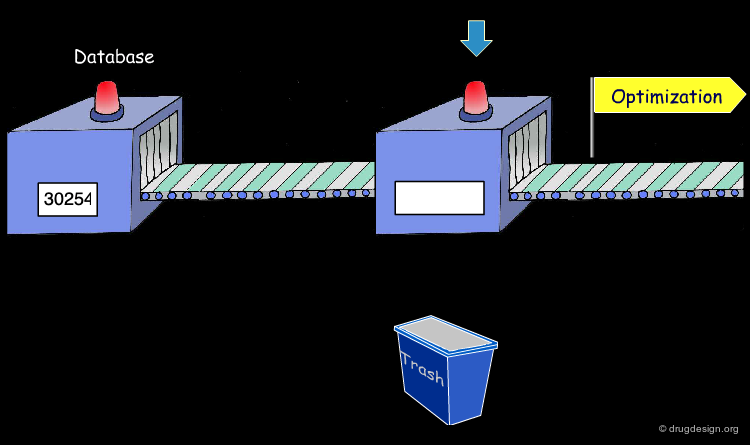
Whether a classical or high-throughput approach is used, the drug discovery process passes through the three steps mentioned here. It typically requires the study of 10,000 or more molecules to find the one that will become a commercial drug. Out of 10,000 molecules explored, only one of them may reach the market.
Starting Molecule in a Project¶
Initial Hit or Lead Compound in drug discovery was centred around natural products but as technologies developed, a wider range of pharmaceutically active compounds were used as the starting point for the development of drugs.
Optimization of the Lead¶
Once a reference structure is available, it is then used as a prototype or "lead compound" for designing more effective therapeutic agents having similar or related chemical structures by exploiting the structure-activity relationships (SARs) observed. This is the so-called optimization process in drug discovery. If it is available, the 3D structure of the target protein can also be exploited to refine the SAR and design better compounds.
Potency and Selectivity¶
The first step in the optimization of a lead is to find molecules with sufficient potency and selectivity. Nanomolar potency in an in vitro assay is desired. Once good candidate molecules are found, the next step is to find a molecule with the best multidimensional properties including all those presented in the following pages.
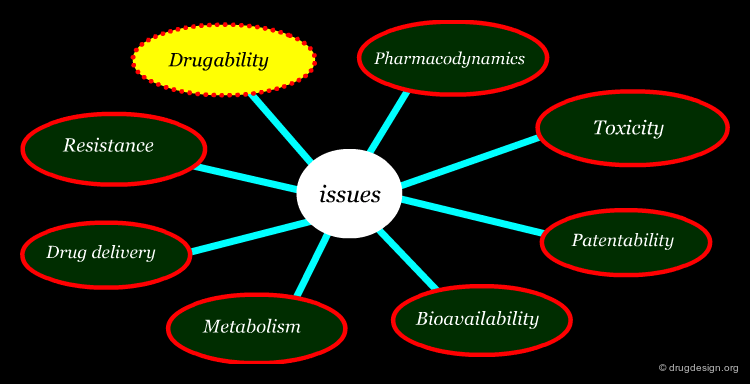
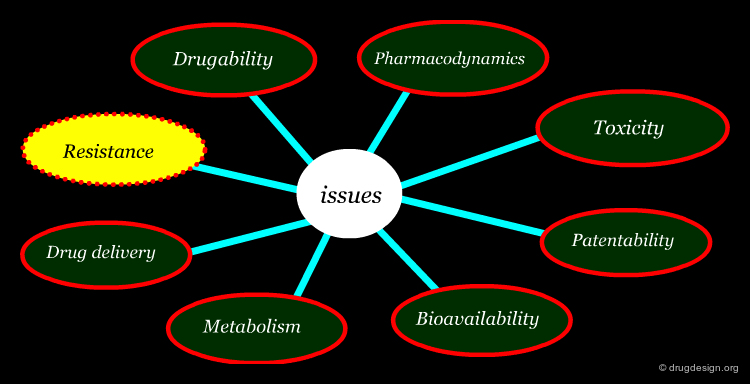
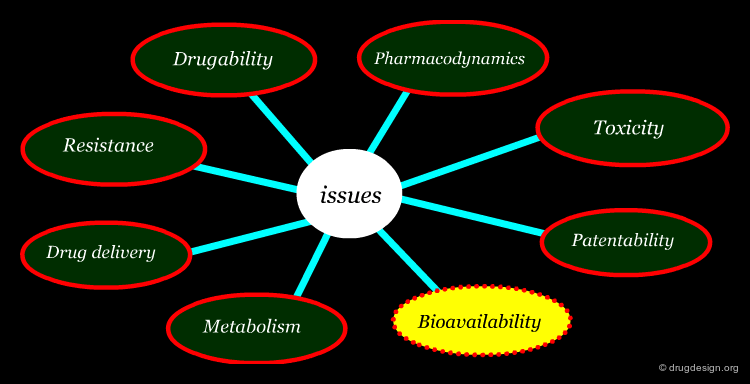
Beyond Potency and Selectivity in Lead Optimization¶
There are many important issues that need to be considered in the optimization of a drug discovery lead to obtain a viable drug candidate. The main ones include: drugability, resistance, pharmacodynamics, drug delivery, metabolism, bioavailability, toxicity and patentability.
Drugability¶
Usually the assessment of the "drugability" of a candidate drug is made by early consideration of its solubility, bioavailability and ADME properties. This is important in the selection of a hit/lead in drug discovery.
Resistance¶
Drug resistance is the reduction in effectiveness of a drug in curing a disease. For example HIV-1/AIDS inhibitors induce resistance where the virus generate mutants that resist the action of the drug. Drug discovery attempts to find drugs that are less susceptible to development of resistance.
Pharmacodynamics¶
Pharmacodynamics (cf. glossary) is important in drug discovery. It describes and quantifies the bodys' response to a drug on the physiological, biochemical and/or molecular level. Together, pharmacodynamics and pharmacokinetics (ADME) considerations can lead to design of molecules and dosing regimens that ensure the drug reaches its site of action with minimal adverse effects.
Drug Delivery¶
"Formulation" is an important aspect of drug discovery. It studies the results of different delivery forms to decide the optimal way for giving the drug to a patient.The more common delivery forms are oral tablets or capsules, oral liquids, topical ointments and injections. There are a vast array of possible dosage forms including transdermal patches, inhalers and intranasal medicines.
Metabolism¶
In drug discovery a good understanding of drug metabolism is essential because one can optimize the metabolic stability of a compound or activate a prodrug and thereby design a better drug. Metabolism is one of the most important determinants of the pharmacokinetic profile of a drug.
Bioavailability¶
Bioavailability describes the fraction of a drug that reaches the systemic circulation. A medication administered intravenously, is 100% bioavailable; however, when it is administered via other routes such as orally, its bioavailability decreases due to incomplete absorption. Oral drugs have to pass through the liver before they can circulate in the blood stream; also the blood-brain barrier prevents many drugs from reaching the brain. Poor bioavailability is very often associated with poor water solubility and the ability for a drug to penetrate membranes is mainly governed by its lipid solubility (and molecular weight).
Toxicity¶
Toxicity is the degree to which a drug is able to damage the exposed patient; every drug has toxicity at sufficient dose. Therapeutic ratio is the efficacious dose vs. the toxic dose. Several types of drug toxicities that need to be under control in drug discovery are presented and discussed in the other chapters of this course.
Patentability¶
Patentability represent an important issue in drug discovery and is presented in the following pages.
Protection of Drug Discovery Achievements¶
R&D based pharmaceutical companies must protect their drug discoveries. Molecules or classes of molecules, formulations containing the molecule(s), process for making the compound, intermediates involved in the process, and uses of the compound(s) for the treatment of a particular disease must be claimed in a patent to protect the discovery. Only through patent protection can the company hope to earn enough to recoup their R&D investment.
Lifetime and Effective Lifetime of a Patent¶
Drug discovery plays a pivotal role in contributing to the extension of the patents of the company. Patent extension is vitally important in the pharmaceutical industry because by the time a drug is marketed as much as 12 years of patent protection may have elapsed. This means that without extension the effective patent term is only 8 years. For example, a new formulation can be used to obtain a patent extension.
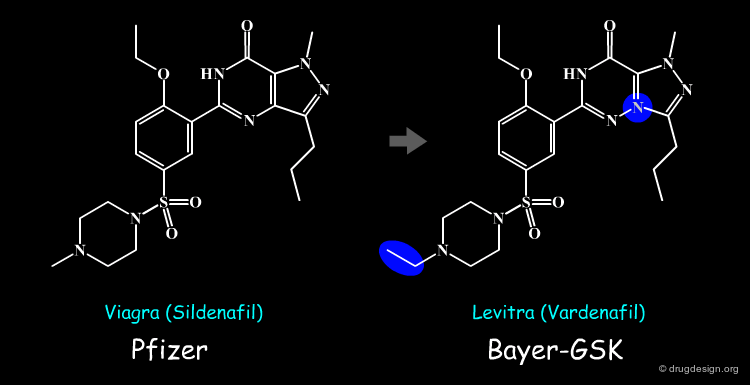
Assessing Patentability: The Viagra-Levitra Example¶
Drug discovery plays an important role in a pharmaceutical company for assessing the patentability of a molecule conceived on the basis of one its competitors. In the example below drug discovery scientists of Bayer-GSK estimated that Levitra would not be considered as an infringement on the Viagra patent. Indeed, Pfizer sued Bayer and GlaxoSmithKline for infringement on their patent and the case failed both in Europe and in the USA.
articles
Vardenafil Bayer Yakuhin Sommer F, Engelmann U. Curr Opin Investig Drugs 3(4) 2002
Comparison of phosphodiesterase type 5 (PDE5) inhibitors Wright PJ. Int J Clin Pract
. 2006
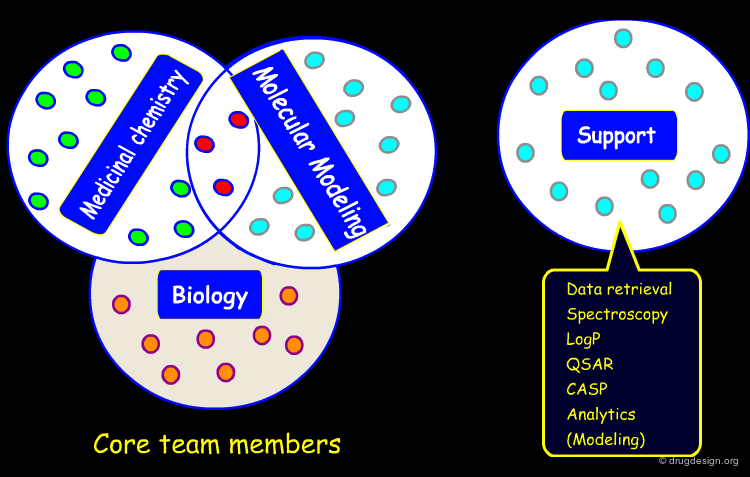
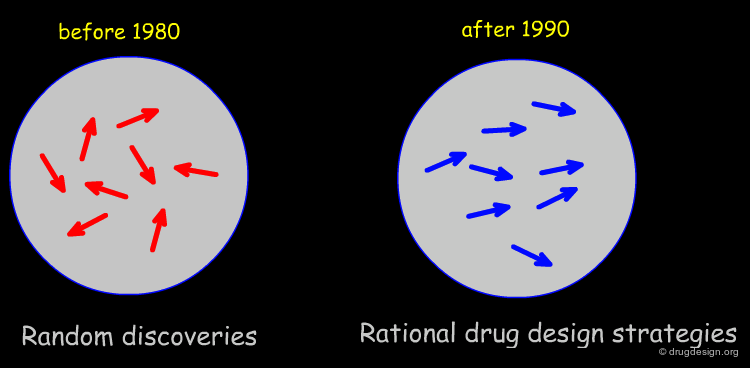
Drug Discovery before and after 1980¶
Up to the 1980's drug discovery was mainly driven by a collaboration between medicinal chemists and biologists. All other disciplines supported the research team with experts contributing as consultants rather than full members of the core drug discovery research unit. In the mid 1980's the situation changed in many companies and the molecular modeler started to be a full member of the core research team.
Intelligent Management Strategy¶
The success of modern drug discovery teams is the result of an intelligent management strategy promoting the integration of the different specialists involved in a project. Before 1980 drug discovery was conducted by individuals resulting in almost random discoveries. After the 1990's the different experts learned to work together in a concerted manner. The principles of rational drug design are presented in the next section.
Rational Drug Design Strategy¶
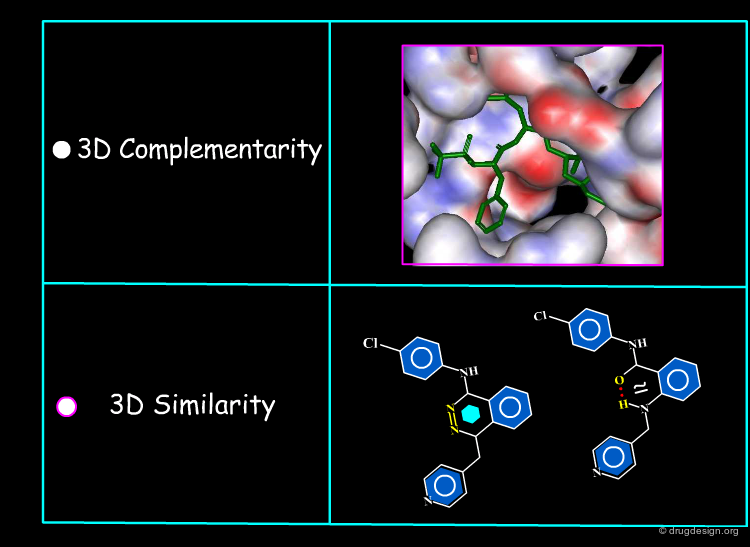
Two Major Approaches¶
Rational drug design is based on a molecular understanding of the interactions between the drug and its target in biological systems. When the 3D structure of the target protein is known, this information can be exploited for the design of new ligands. When the three-dimensional structure of the target protein is not available, one can exploit the information provided by known active molecules. The interaction with the target protein remains the central issue, however the design is made indirectly.
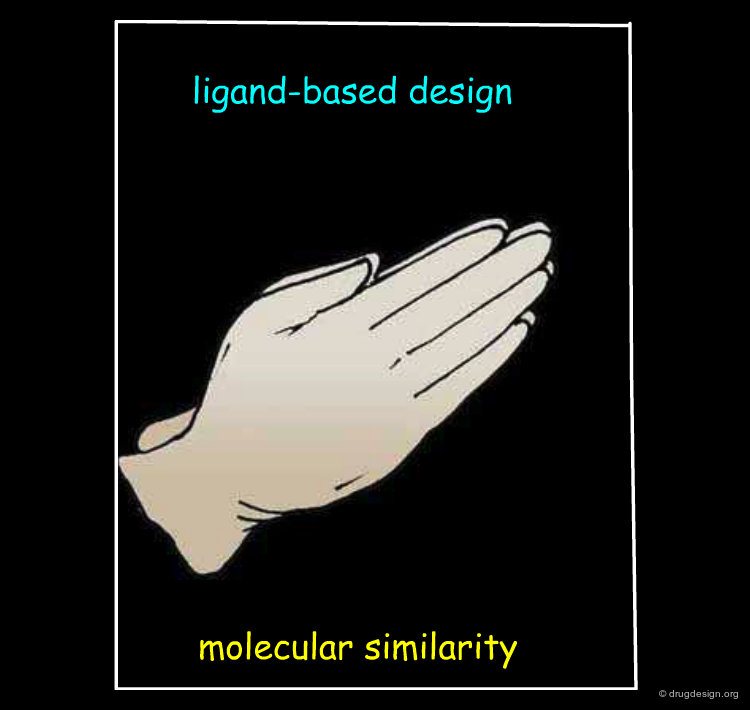
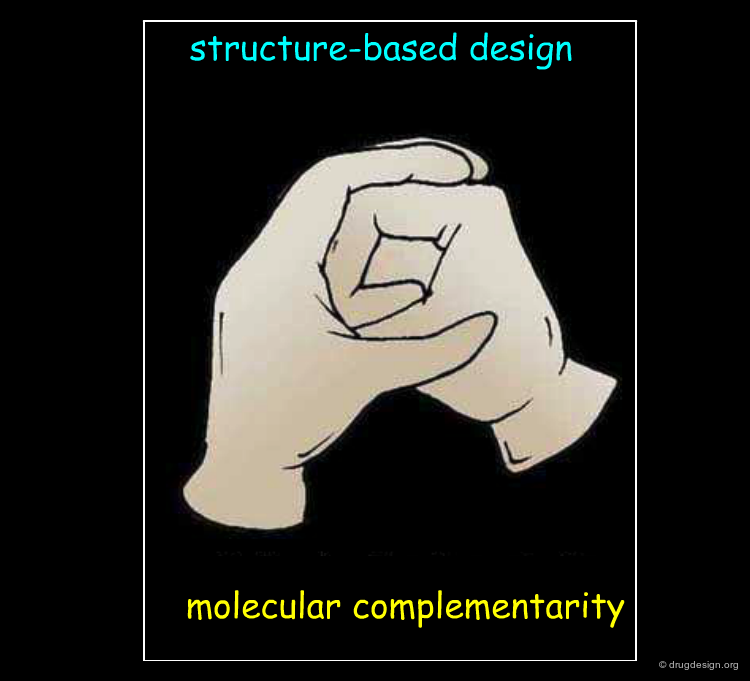
Underlying Principles¶
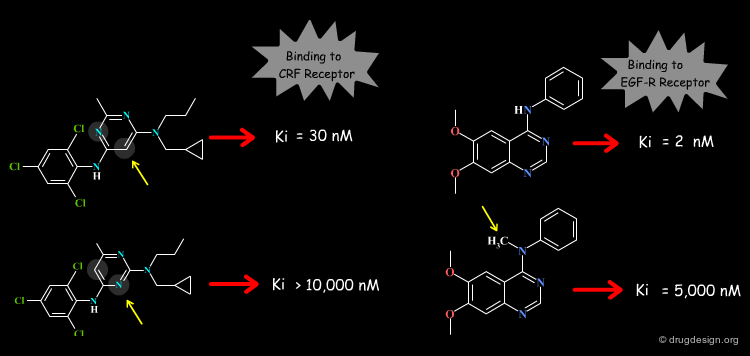
Structure-based drug design exploits the detailed 3D recognition features between a ligand and its receptor, and the underlying concept is "molecular complementarity". Ligand-based drug design exploits the likeness between molecules that is expressed in terms of the concept of "3D molecular similarity".
Contribution of Molecular Modeling¶
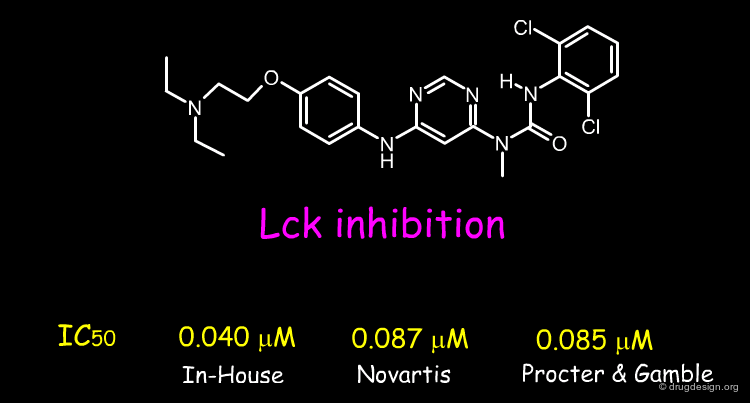
Computer modeling has greatly contributed to the success of rational drug design technologies in drug discovery. It helped to motivate the research teams and to reduce the number of molecules to synthesize in order to find a lead compound. Success stories cannot be anymore presented in one single review because of the great number of examples.
The Molecular Similarity Principle¶
In drug discovery the "molecular similarity principle" is the underlying concept of virtually all ligand-based drug design methods. It assumes that similar molecules tend to behave similarly, while more dissimilar molecules exhibit more distinct properties.
articles
Chemical Similarity Searching P. Willett. J. Chem. Inf. Comput. Sci. 38 1998 10.1021/ci9800211
Approaches to Measure Chemical Similarity N. Nikolova and J. Jaworska. QSAR Comb. Sci. 22 2004 10.1002/qsar.200330831
Molecular similarity: a key technique in molecular informatics A. Bender and R.C. Glen Org. Biomol. Chem. 2 2004 10.1039/b409813g
book
M.A. Johnson and G.M. Maggiora. Concepts and Applications of Molecular Similarity Wiley 1990
The Molecular Complementarity¶
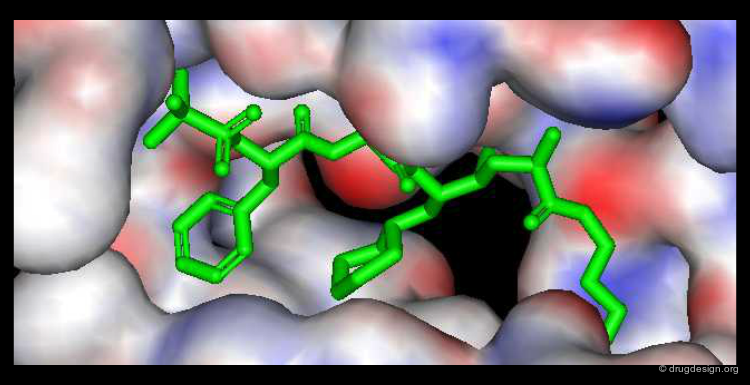
When the 3D structure of the target protein is available, the molecular complementarity can be easily seen. The principal forces involved upon the binding of a ligand to a biological system are: hydrophobic interactions, hydrogen bonding and electrostatic forces. Energy dictates molecular associations between a protein and a ligand and controls all these interactions: they are exploited in drug discovery to maximize the binding of a candidate molecule.
The Concept of 2D and 3D Pharmacophores¶
One of the most powerful concepts used in drug discovery is the principle of pharmacophores. These are considered as 2D or 3D entities carrying the structural elements that are needed to produce the desired biological action.
Structure-Based Drug Discovery: The Aliskiren Example¶
With the aim of developing develop an antihypertensive drug acting as renin inhibitor Novartis used a 3D homology model of the enzyme to predict the binding mode of a potent reference peptide. This complex served as a basis for the design of orally available non-peptidic inhibitors. This led to the discovery of Aliskiren (Tekturna), a drug now used for the treatment of high blood pressure. Molecular modeling played a central role in the discovery and development of this drug for which the team received the prize "2009 Heroes in Chemistry" from the American Chemical Society.
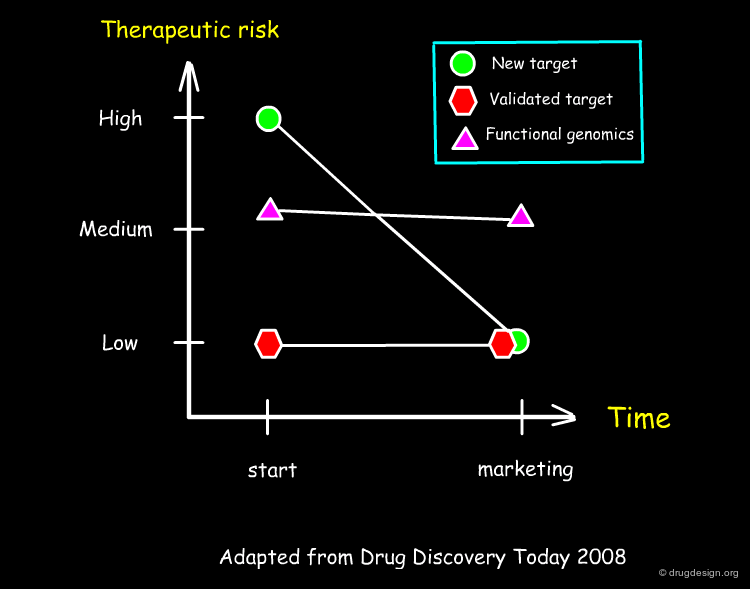
Therapeutic Risk in a Project¶
Drug discovery is driven by innovation and competition. Some projects are very risky because the corresponding target remains to be validated. Less risky projects are those based on the copy of an existing drug having a validated target. From the managerial point of view both approaches remain valid and in both cases scientists involved in drug discovery know exactly what to do.
The Major Players in Drug Research¶
Role of Each Scientist¶
Drug discovery develops within a mutidisciplinary framework. Some of the tasks of the major players done by each scientist in drug research are presented here. The list is far from being exhaustive, it just intends to provide some familiarity with the everyday activity of each of these experts. Note that, in small groups, many of these functions may be assumed by the same scientist.
Organic Chemist¶
The organic chemist is often the project leader of a drug discovery project. The major roles of the organic chemist include the following: prepare new molecules, analyze the SAR data, order new molecules, design a library of molecules, scale up a chemical synthesis.
Prepare new Molecules¶
In the first place the organic chemist prepares new molecules. He must find the best synthetic scheme to achieve this goal as quickly as possible. When they are ready, the molecules are sent to the biologists for biological testing.
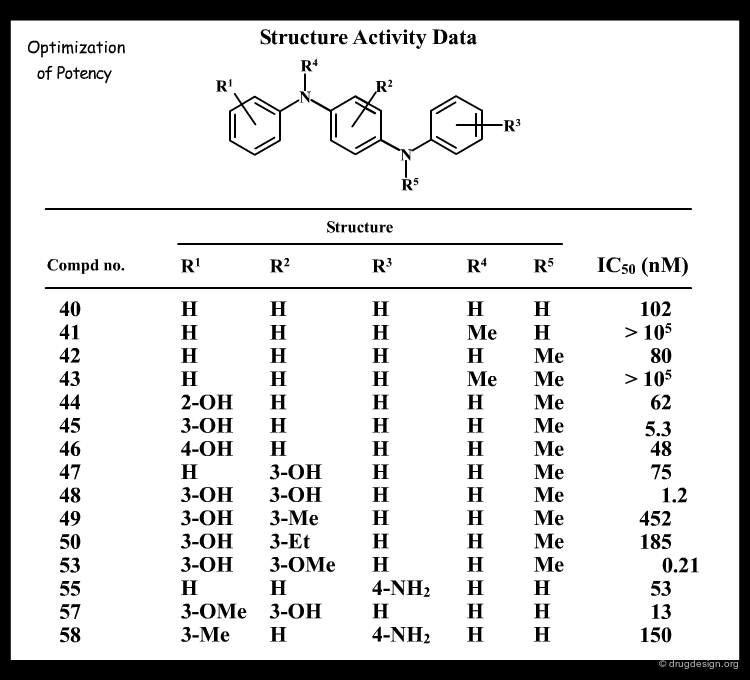
Analyze SAR Data¶
Below is shown an example of "digested" information generated by the organic chemist. It is based on the data accumulated in the research project concerned.
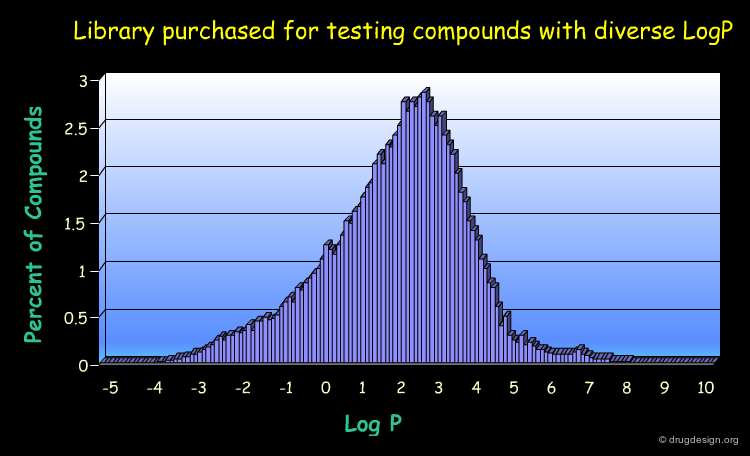
Order new Molecules and Libraries¶
Many molecules can be purchased from commercial companies. The advantage of using them is that they can be purchased and tested rapidly. This may accelerate the lead discovery process. In the library below, the LogP diversity is considered.
Design of a Library of Molecules¶
The chemist designs a combinatorial chemistry program to prepare a library of either focused or diverse molecules. He then passes the products that were made to the biologists for being tested.
Media
Scale Up a Chemical Synthesis¶
When a lead candidate or a candidate drug has been identified, the organic chemist works to scale up the synthesis. He modifies the synthetic scheme or the reagents to reduce the costs of the preparation and tries to increase the effectiveness of a given step by changing some of the experimental conditions such as the reagents, the temperature, the time or the catalysts.
Media
Actelion Pharmaceuticals Ltd. Reproduced from Actelion, with permission Actelion
Biologist¶
The biologist works closely together with the medicinal chemists. The major roles of the biologist include the following: develop biological assays, develop in-vivo models for testing molecules, test new compounds, compare the results obtained by other groups, develop models for early assessment of toxicity, implement a platform for high throughput screening.
Develop Biological Assays¶
He develops a biological assay for measuring the biological activity of a substance by comparing its effects with those of a standard preparation on a test organism. He can replace the assay by measuring the concentration of the active principle using an analytical method (e.g. radioimmunoassay). In case the target protein is well characterized, he develops a ligand binding assay to identify the presence of a molecule, to quantify the assay by measuring the ligand activity.
Develop in-vivo Models for Testing new Molecules¶
The biologist works with whole animals; he is responsible for demonstating the relevance of the in-vivo model for the target disease. He prepares pharmacological in-vivo models for testing and assessing new molecules and for the development of improved drugs.
Test New Molecules¶
In the first place the biologists test molecules given to them by the chemists. The tests can be performed in-vivo or in-vitro. In-vivo tests use a whole living organism, whereas in-vitro tests are performed in a controlled environment, such as in a test tube or Petri dish. The results are generally given as a structure-activity table showing the molecules and their biological activities.
Compare Results Obtained by Other Groups¶
Whenever possible, biologists compare the results obtained with their biological tests with the values obtained by other groups, on a given molecule. Availability of similar molecules in a given class is a great help allowing risk to be more objectively compared.
Develop Models for Early Assessment of Toxicity¶
Many drugs are abandoned in the clinical trials because of their toxicity. The biologist develops assay protocols to provide essential levels of information required for early assessment of compound toxicity in a drug discovery project.
Media
Implement Platform for High Throughput Screening¶
High-Throughput Screening allows to quickly test millions of molecules. This technology widened considerably the possibilities in drug discovery (e.g. a run can contain from 100,000 to more than 2,000,000 compounds.)
Molecular Modeler¶
The medicinal chemist is the principal partner of the molecular modeler. His major role includes the following: use a variety of computer graphics and computational tools to build structure-activity models, provide structural models of good quality, analyze and suggest candidate molecules, assess molecules submitted by the team, explore ways for 3D alignment of molecules.
Provide Structural Models of Good Quality¶
The first issue in a project consists of having a good model of the target protein, of complexes with other molecules (substrate, molecules of the competitors ...). The models created by the molecular modeler are communicated to the members of the research team. They can analyze them on their computer and explore new ideas for designing other molecules.
Analyze and Suggest Candidate Molecules¶
Most of the time the molecular modeler analyzes alone the molecular structures and explore how they may be exploited. Regular meetings are then organized with the members of the research team where the modeler shows his results, discuss possibilities and decide with the team what should be done next, until another session takes place to move forward in the project.
Assess Molecules Submitted the Team¶
Molecules are submitted by the team to the modeler for considering their validity according to the desired 3D requirements. When the molecules do not conform to these requirements and taking into consideration that the chemist knows how to synthesize such products, the modeler looks for structural modifications that may lead likely bio-active structures.
Explore Ways for 3D Alignment of Molecules¶
When two molecules have similiar biological properties and are chemically unrelated, it is not always easy to find their common 3D features. The molecular modeler analyzes the conformational posibilities of the molecules considered and then searches for ways to align them in 3D.
Biotechnologist¶
The biotechnologist interacts with the biophysicians, the chemists and the modelers. He aims at providing experimental models that can be used by all the members of the teams. His major role includes the following: clone DNA sequences, isolate an enzyme, identify a disease-relevant target and validate a new assay.
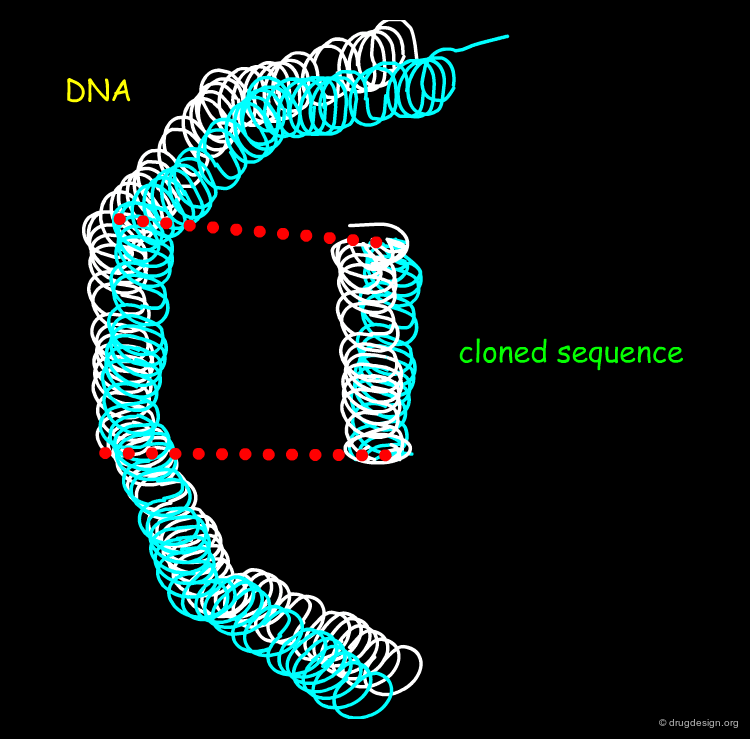
Cloning DNA Sequences¶
In order to clone and amplify a fragment of DNA the biotechnologist inserts the DNA material into a vector. This technique is of paramount importance in drug discovery in providing the means for making quantities of the corresponding recombinant protein that can be then crystalized for X-ray determinations.
Isolation of Enzyme¶
Several methods have been developed to isolate and purify enzymes from aqueous multi-phase systems containing polymeric materials. Protein extraction kits are also commercially available, which are used by the biotechnologist in many drug discovery projects.
Media
Identify Disease-Relevant Target¶
The biotechnologist develops high-resolution purification methods enabling him to analyze and search for proteome matches for all the proteins of the sample he is analyzing. A typical proteomic experiment involves the analysis of complex samples with electrophoretic gels and liquid chromatography.
Validate a New Assay¶
Validation of an assay method is performed on a case-by-case basis to ensure that the parameters are appropriate for the method intended to be used.
Computational Chemist¶
The computational chemist interacts with the chemists and the modelers. His major roles include the following: search for a predictive 3D-QSAR model, launch high throughput docking simulations, estimate the binding energies of new molecules, assess the quality of QSAR models.
Search for a Predictive 3D-QSAR Model¶
When a set of molecules have similiar biological properties and are chemically unrelated, the computational chemist tries to develop a 3D-QSAR model enabling to explain their activities that can be used to predict the biological properties of new molecules.
Launch High Throughput Docking Simulations¶
The computational chemist launches high throughput docking simulations in order to identify both interesting ligands and key interactions that can be exploited in the target protein.
Estimate the Binding Energies of New Molecules¶
The theoretical prediction of the binding energy of a system is of central importance for understanding biological mechanisms and for designing new molecules. The method is based on free energy perturbation calculation and requires substantial molecular dynamics treatments. The method is used case by case by the computational chemist.
Assess the Quality of QSAR Models¶
Many computer programs include the option of generating QSAR models however, the users that exploit this functionality are not always aware of the exact statistical significance of the models generated, in particular when multiple solutions are proposed. The computational chemist contributes to identify good models that can be further exploited to predict in a reliable manner the activity of new molecules not included in the training set.
Structural Bioinformatician¶
The structural bioinformatician is a specialist in macromolecules. His major role includes the following: develop tools for encoding and visualizing biomolecules in a given project, understand protein flexibility, create 3D models of new proteins, decode the function of a protein, reveal key residues in a protein.
Encoding and Visualizing Biomolecules¶
The structural bioinformatician encodes and visualizes biomolecules in a way that the displayed information is informative and of high informational content for the drug discovery project concerned.
Understand Protein Flexibility¶
The structural bioinformatician spends time to study protein folding. The crystallographic data available in the protein databank is of central importance for such analyses. Folds can be analyzed, classified and modeled. Understanding protein flexibility is of great importance in drug discovery and also it is a complex issue that cannot always be modeled.
Create 3D Models of new Proteins¶
The structural bioinformatician develops homology models of proteins of interest until experimental X-Ray data become available. The models can be used by the research team to understand SAR and to design new molecules.
Decode the Function of a Protein from its 3D Structure¶
Structural bioinformaticians aim at understanding the function of a protein from its 3D structure. Such analyses progressively contribute to the development of predictive methods that generate models that can be fully exploited in drug discovery.
Reveal Key Residues in a Protein¶
The alignment of similar proteins is made by the structural bioinformatician; he can reveal which residues of a protein are essential and those that are not important. This type of analysis generates knowledge of great importance for the drug discovery team.
Cheminformatician¶
The cheminformatician is a specialist of small molecules. His major role includes the following: calculate molecular properties, find similar molecules, analyze HTS results, contribute to virtual screening and library design, update and maintain databases.
Calculate Molecular Properties¶
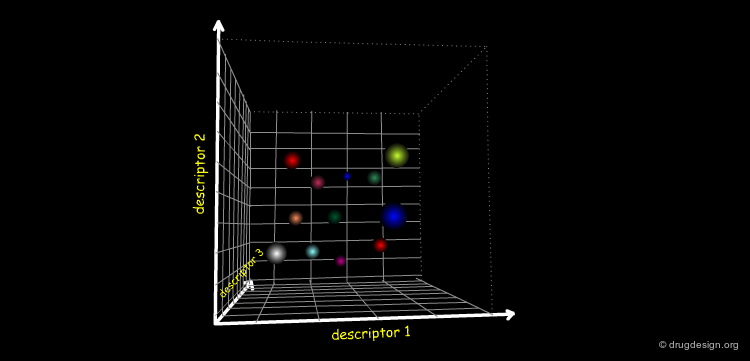
The cheminformatician calculates the molecular properties of compounds and compares them. He can exploit them for many purposes; in particular, he identifies descriptors that are relevant for the biological properties of molecules. He can predict the biological activities of new compounds.
Find Similar Molecules¶
The cheminformatician finds similar compounds with chemically unrelated 2D structures. To do so, he represents the molecules in the space of their main descriptors. The molecules can be tested and may lead to the discovery of novel prototype structures.
Analyze HTS Results¶
The cheminformatician analyzes the results of a HTS campaign and identifies hits of potential interest.
Contribute to Virtual Screening and Library Design¶
The cheminformatician undertakes virtual screening analyses and collaborates with the organic chemist in the application of a library screening program aiming at the discovery of new compounds.
Update and Maintain Databases¶
The cheminformatician continuously updates and maintains the content of the databases. These databases are continuously interrogated by the members of the drug discovery teams.
Biophysicist¶
A biophysicist can be expert in X-Ray crystallography, NMR spectroscopy or in cryo-electron microscopy. Each field is generally managed by a different person but they all contribute to the establisment of 3D models of the proteins involved in drug discovery projects.
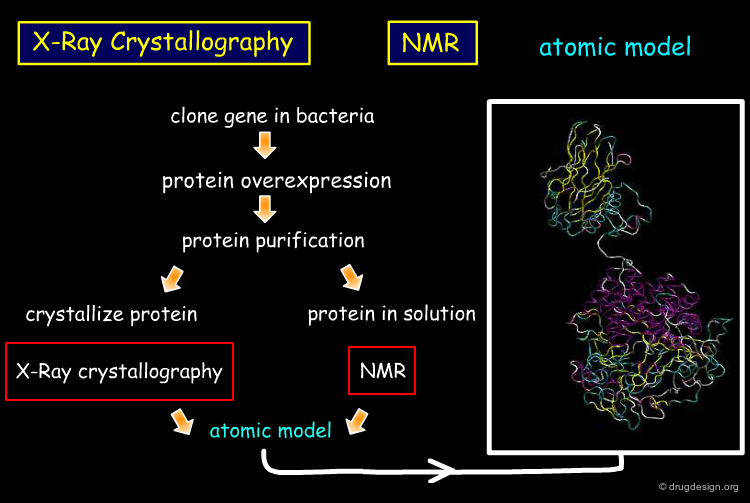
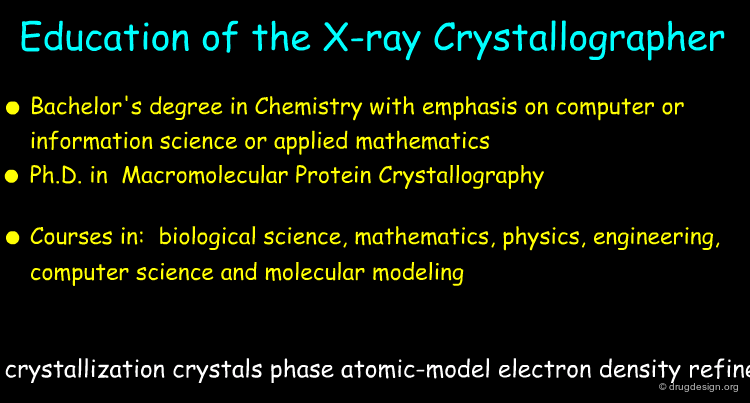
X-Ray Crystallographer¶
The major roles of the X-Ray crystallographer include the following: prepare crystals of a protein or a complex with a protein, measure diffraction pattern and density maps, fit the electron density map, refine the 3D atomic model.
Prepare Crystals of a Protein or a Complex¶
Preparing protein crystals suitable for structural analysis is currently the bottleneck in structure determination by X-ray crystallography. One method used by the X-Ray Crystallographer is to start by getting few crystals to grow in the highly supersaturated solution. Then the crystals are exposed to a less saturated solution so they can grow. This is done either by moving the crystals or changing the saturation of the solution.
Media
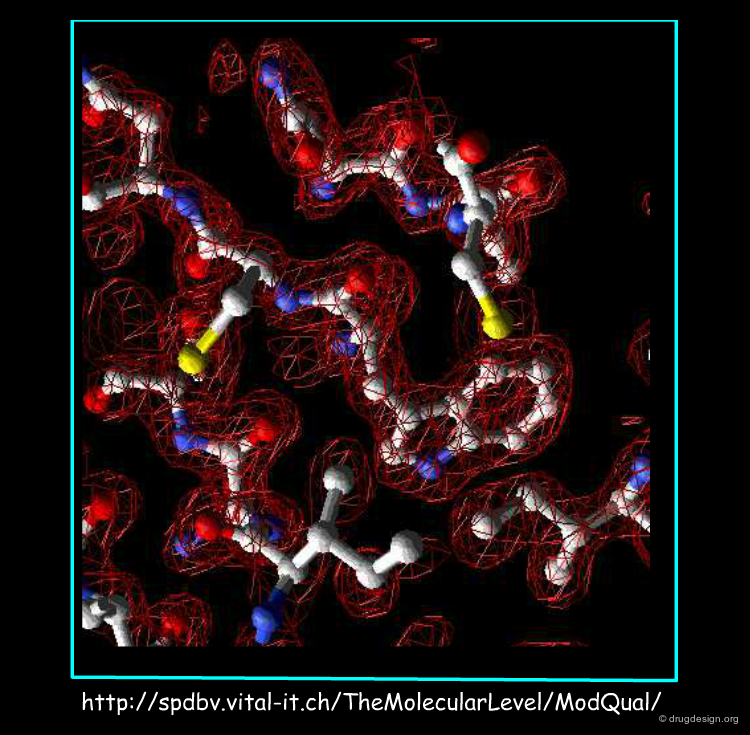
Diffraction Pattern and Density Map¶
Electron density map construction requires 3D Fourier transformation computations and the resolution of the so-called "phase problem". This step is done by the X-Ray crystallographer.
Media
Fit the Electron Density Map¶
Then, the crystallographer uses the atomic positions of his initial model to fit and adapt to the observed diffraction data.
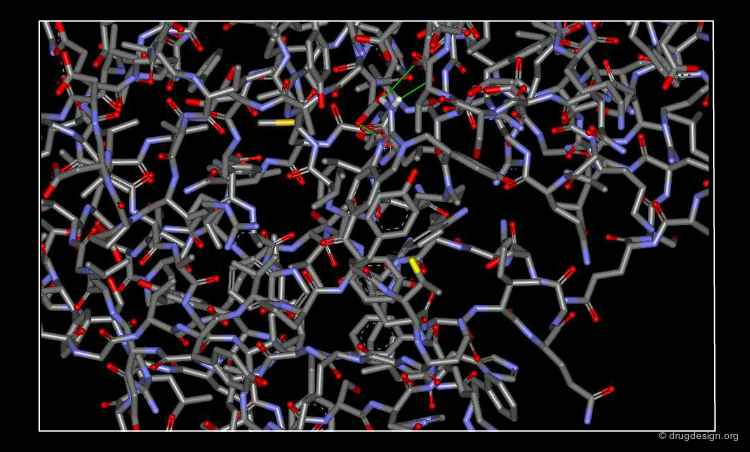
Refine the 3D Atomic Model¶
The crystallographer aims at maximizimg the correlation between the diffraction data and the current model. This is an iterative process and the agreement is measured by the so-called "R-factor". When convergence is achieved the crystal structure can be considered as solved.
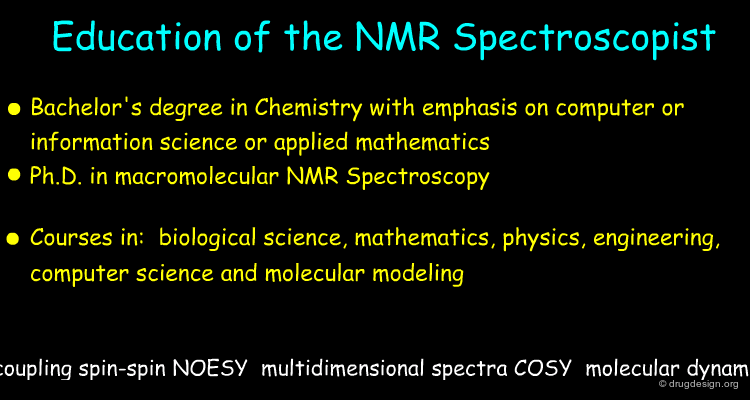
NMR Spectroscopist¶
The major roles of the NMR Spectroscopist are similar to those of the X-Ray crystallographer. Contrary to X-Ray crystallography, the NMR structure determination is made in solution. The spectroscopist role includes the following: prepare a solution of the protein or a complex, collect and analyze the chemical shifts, construct a preliminary model that fit the chemical shifts and refine the 3D atomic model resulting in a set of plausible conformers.
Chemistry in Drug Discovery¶
Chemistry in Drug Discovery¶
Chemistry plays a central role because of its ability to create and produce molecules for drug discovery. The chemist is one of the main drivers of the drug discovery process.
Synthesis of Complicated Molecules¶
Complicated natural molecules can be prepared by total synthesis, and this is of great importance in drug discovery. Synthetic chemistry opened the way of making by total synthesis molecules that are found in nature only in small quantities, but it also opened the possibility of making analogs of these natural compounds to find those with the most interesting biological potential.
articles
Stereospecific total synthesis of cortisone Sarett LH, Arth GE, Lukes RM, Beyler RE, Poos GI, Johns WF and Constantin JM J. Am. Chem. Soc. 74 1952
Total synthesis of taxol Nicolaou KC, Yang Z, Liu JJ, Ueno H, Nantermet PG, Guy RK, Claiborne CF, Renaud J, Couladouros EA, Paulvannan K, et al Nature 367(6464) 1994
Three Methods in Synthetic Chemistry¶
There are three main avenues in chemistry for the synthesis of new molecules for drug discovery: the classical, the parallel and the combinatorial chemistry methods.
articles
Combinatorial compound libraries for drug discovery: an ongoing challenge Geysen HM, Schoenen F, Wagner D and Wagner R Nat Rev Drug Discov. 2(3) 2003
Drug discovery by dynamic combinatorial libraries Ramstrom O and Lehn JM Nat Rev Drug Discov. 1(1) 2002
Knowledge-based approaches in the design and selection of compound libraries for drug discovery Viswanadhan VN, Balan C, Hulme C, Cheetham JC and Sun Y Curr. Opin. Drug Discov. Devel. 5(3) 2002
Classical Drug Discovery¶
The classical method consists of the preparation of a single molecule. Whatever synthetic scheme he considers, the chemist aims at the preparation of one well-defined compound from a few milligrams to a few grams of the target substance. The purified molecule is then tested by the biologist.
Parallel Drug Discovery¶
Recent developments in robotics for synthetic chemistry allow the automated preparation of many compounds involving different reactions "in parallel". The biological tests are made either on purified compounds or on mixtures.
Combinatorial Drug Discovery¶
Many analogs of a given scaffold can be synthesized with combinatorial chemistry. One part of a molecule is maintained, as different chemical groups are attached to its molecular framework to produce a series of similar molecules. Only a few milligrams of compounds can be obtained by this method, however this is generally sufficient for primary biological screening.
Chemistry in Lead Discovery¶
Not all lead molecules ultimately become drugs. Medicinal chemistry is fully exploited to transform a "hit" with poor activities into a lead drug candidate, and this is one of the most important aspect of drug discovery.
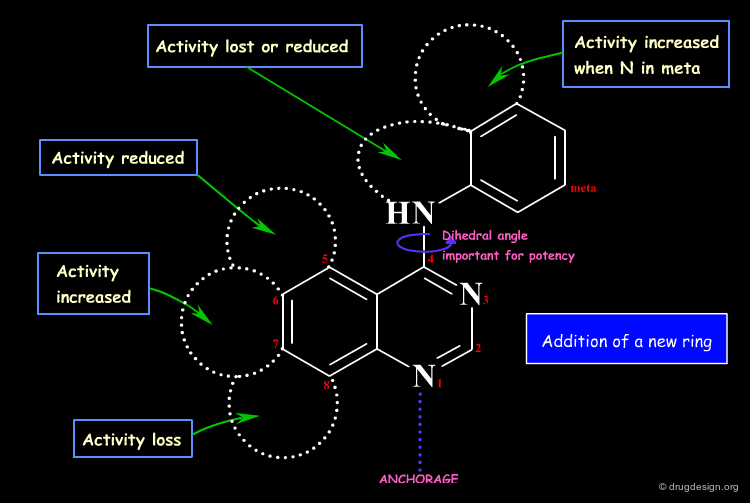
Protein Kinase Example¶
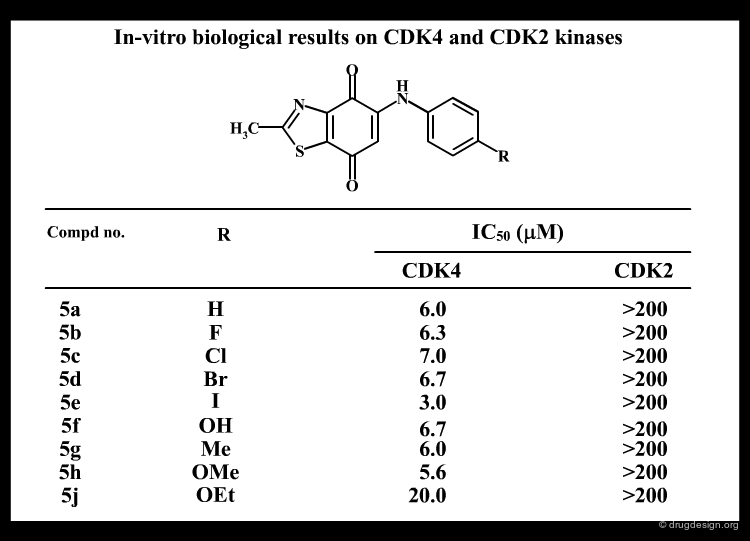
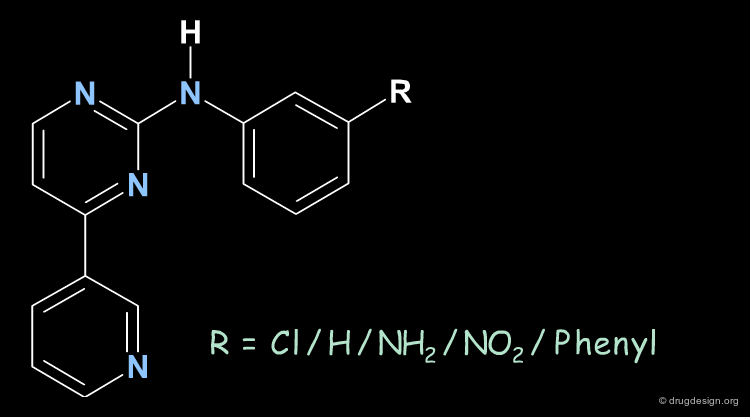
The following example illustrates an effort undertaken in the research of inhibitors of the Bcr-Abl tyrosine protein kinase for the treatment of chronic myelogenous leukemia (CML), and the optimization of a compound by screening. The chemistry program revealed the importance of the substitution (in the meta position) of the phenyl ring, which greatly influenced the potency and the selectivity of the analogs.
Lead Optimization¶
Lead optimization is deployed to identify the best analog of an initial lead compound. To do so, a great number of analogs are prepared and their biological properties examined. The most promising drug candidate is then selected for development.
articles
The combinatorial synthesis and chemical and biological evaluation of a 1,4-benzodiazepine library Bunin BA, Plunkett MJ and Ellman JA Proc. Natl. Acad. Sci. 91 1994
Example: Optimization of the Gleevec Series¶
Many analogs of the following lead scaffold were synthesized and tested. The molecule STI571 appeared to be the best member of the series, having good activities, selectivity and safety properties. This drug candidate entered into development and is now commercialized for the treatment of CML cancer (Gleevec).
articles
Potent and Selective Inhibitors of the ABL-Kinase: Phenylamino-pyrimidine (PAP) Derivatives J. Zimmermann et al. Bioorganic and Medicinal Chemistry Letters 7 (2) 1997
Chemistry in Drug Development¶
When a candidate drug is selected for development, the existing synthetic scheme may need to be reconsidered from a bulk production perspective that takes into consideration additional factors such as the price of the synthesis, the protection against hazards and respect for the environment.
The Present and Future Face of Drug Discovery¶
Outsourcing: an Important Cultural Shift¶
Pharmaceutical companies are currently outsourcing about one fourth of their R&D expenses. It spans the entire R&D activity from combinatorial chemistry to clinical trials. This important cultural shift proved to reduce the heavy investment in infrastructure which would normally be necessary for the company.
Why Outsourcing Develops so Well?¶
Company mergers and acquisitions have slowed decision-making and delayed many important mechanisms in the pharmaceutical companies. This prompted pharma management to intensify clinical trial outsourcing to compensate for this lack of efficiency.
Management Response to Challenging Situations¶
A number of important issues have great impact on the way that drug discovery develops in a pharma company. There is a lot of strain on the management of a company for maintaining the drug portfolios. Pharmaceutical companies have to be prepared to tell what to do when disaster strikes, when a new area must be developed, when a patent expires, when an acquisition must be made, when a late stage failure occurs.
Management Challenges¶
Much more than in the past the management of a pharmaceutical company must be adaptive and able to move the company ahead even under the most difficult circumstances. The managers are juged on their ability to ensure a rich and a varied pipeline developed alone or with partners.
Media
Mergers and Mega-Mergers¶
Experience shows that reliance on known therapeutic agents and serendipity are no longer sufficient to provide a steady pipeline. Pharmaceutical companies find it difficult to sustain desired revenue growth rates. Blockbuster expirations exert intense pressure on them. Mergers or acquisitions is the approach used to overcome some of the difficulties.
Drug Discovery and Mergers¶
As far as drug discovery is concerned, there are good and bad R&D mergers. Of central importance is the innovation issue and the merger may not solve the fundamental problem regarding innovation.
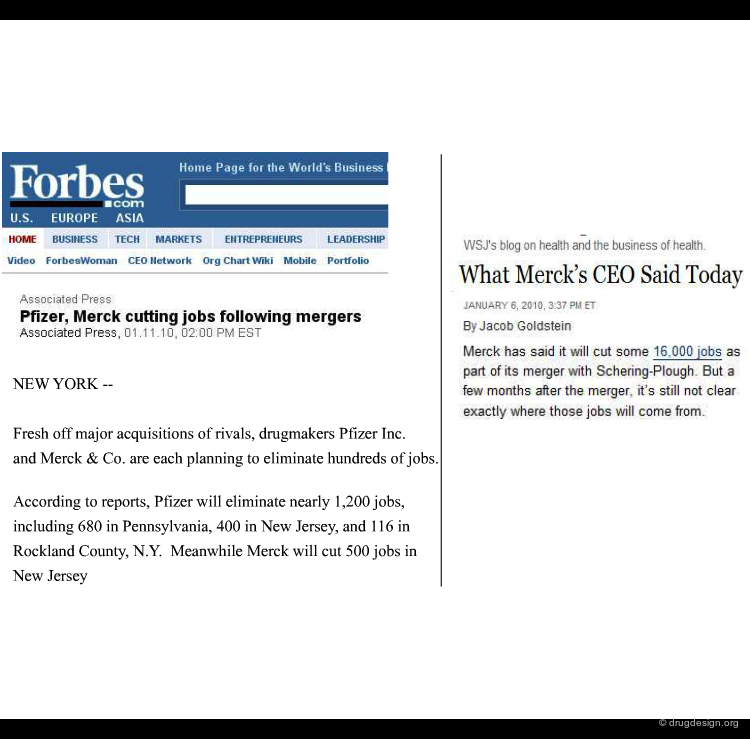
Massive Cut of Jobs Following Mergers¶
Cut of jobs often follow mergers and senior managers are sometimes eliminated. Firing drug discovery scientists may be highly detrimental to the innovative capacity of the companies.
Headcount are not always Reduced¶
Headcount may not be reduced. Further to the conflicts which can arise as a result of conflicting cultures between the companies managements feel that it pays to dismiss existing employees and hire new workers who are not used to the old ways of doing things.
Media
Creation and Development of Startup Companies¶
The layoff of R&D employees can encourage employees involved in drug discovery to create their own company and pursue a project that has been started by them in the original pharmaceutical company. Examples of startup companies with different backgrounds are presented in the following pages.
Startup Example-1: Amgen¶
Amgen was created in 1980 by a group of US scientists and venture capitalists for developing products based on recombinant DNA. Ten years after its creation the company had no sales at all ! Today, it employs 14,000 staff members with annual revenues of more than $14 billion. The company's first products on the market are Epogen (anti-anemia protein) and Neupogen (protein to treat neutropenia); they were the two most successful biopharmaceutical products at the time of their respective releases.
Startup Example-2: Actelion¶
Actelion was created in Basel in 1997 by former co-workers of Hoffman-La-Roche, Switzerland. In 2010 the company employs 2174 coworkers and commercialize three drugs: Tracleer (an oral dual endothelin receptor antagonist for pulmonary arterial hypertension), Zavesca (a small molecule therapy for the treatment of type 1 Gaucher disease), and Ventavis (an inhaled formulation for the treatment of pulmonary arterial hypertension). Actelion's lead product is Tracleer that generated sales of 1,2 billion SFR in 2007.
Startup Example-3: Speedel¶
Speedel was a Swiss-based biotech development company created in 1998 by former coworkers of Novartis (Ciba-Geigy). The renin inhibitor Aliskiren was out-licenced by Novartis in 1999 to Speedel who carried out the Phase 1 and 2 clinical trials. Further to the good results obtained, Novartis exercised its call-back option and took over the subsequent development of Aliskiren (2002). The molecule was commercialized in 2007 and finally, Novartis acquired the entire Speedel company (2008).
Increasing Importance of Generics¶
Once the patent of the drug of a company has passed (up to 17 years) any other company can manufacture and sell the drug with the same ingredients. Thousands of generic drugs are available today. Every year, dozens of blockbuster products come off patent which provides attractive opportunities for generic companies to expand their drug portfolio.
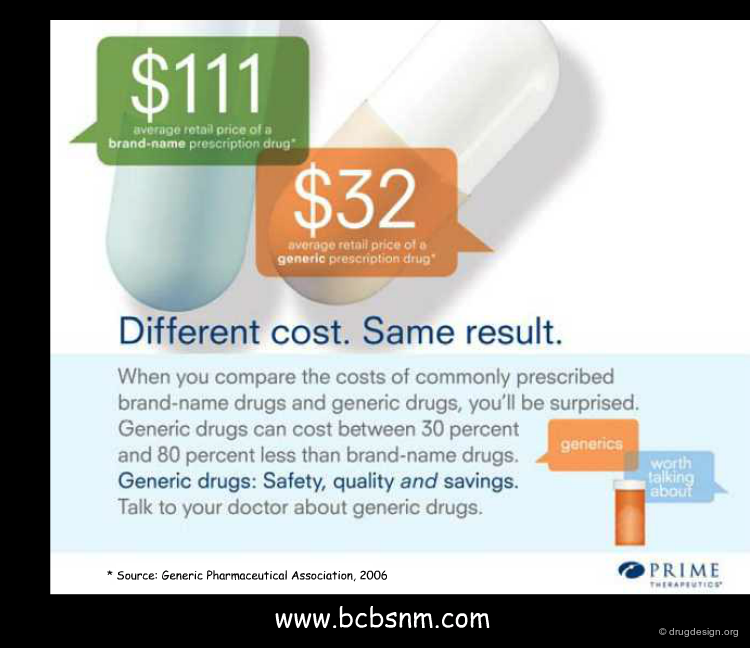
Economical Implications of Generics¶
Generic drugs unable important savings (limited research costs for the generics company) ranging from 30% to 80%. This can save patients, health organizations and insurance companies substantial costs. The generic industry is growing at more than 7.8%, a speed that is faster than the regular pharmaceutical market.
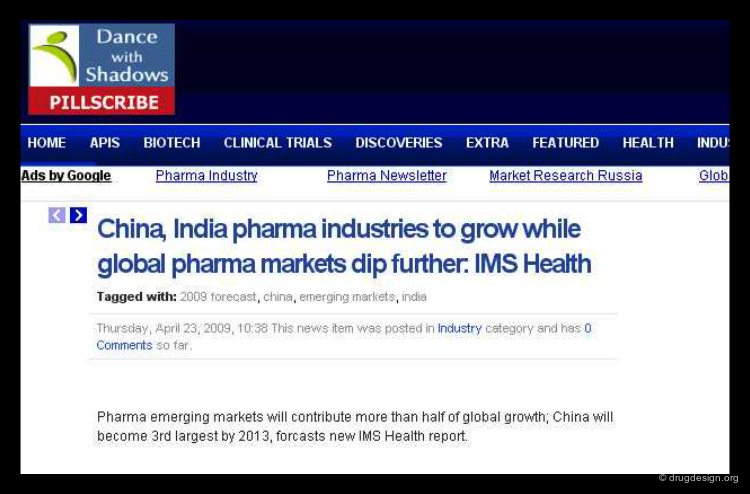
Emergence of New Industrial Players¶
The world economy has substantially changed in the recent years with the emergence of new markets in countries with rapid growth and industrialization. According to the latest IMS Health report, China, Brazil, India, South Korea, Mexico, Turkey and Russia are expected to bring important innovations in drug discovery and sustain an average 40 percent contribution of the global market growth. China and India represent the two most important ones.
Pharmaceutical Industry in India and China¶
The top 10 pharmaceutical companies in India include Ranbaxy Laboratories and Dr. Reddy Laboratories. In China, the pharmaceutical industry is one of the leading ones in the country however the domestic pharmaceutical market is highly fragmented with around 3,000 to 6,000 domestic manufacturers.
Personalized Medicines¶
The development of personalized medicines ("pharmacogenomics") is not science fiction but has taken off. Now that the human genome can be fully decrypted and analyzed in terms of its implications with diseases and drug action, we can expect spectacular progress in the field.
Pharmacogenomics Example¶
Severe or fatal myelosuppression has been associated with an inherited deficiency in thiopurine S-methyltransferase (TPMT). Before starting a therapy with mercaptopurine, the TPMT levels of the patient are measured to be sure that there are no risks of drug-induced myelosuppression due to impaired clearance of the drug (the induced adverse event can be fatal).
articles
Personalized Drug Therapy: The Era of Pharmacogenomics Howard L. McLeod and Janelle M. Hoskins
A Program of the AGA institute April/May 2007
Stem Cell Therapy¶
A completely new type of therapy is now emerging based on the capability of stem cells to be grown and transformed into specialized cells. In particular, this discovery has the potential to regenerate damaged and diseased organs but it also raises ethical issues concerned with the culture and use of stem cells derived from human embryos that has not been fully addressed. Areas where stem cell therapy can be applied include the following.
Some Facts and Figures¶
In the following pages are presented data with direct or indirect implications with drug discovery. The examples are far from being exhaustive but they give some idea on drug discovery contribution to pharmaceutical R&D.
The Cost of Developing a New Drug¶
Compared with other branches of industry, the pharmaceutical industry invests a great deal of money in research and development. With time, the cost of developing a new medicine has grown exponentially although innovation in drug discovery is progressing at lower speed.
articles
The Cost of Biopharmaceutical R and D: Is Biotech Different? J. A. DiMasi and H. G. Grabowski et al. Managerial and Decision Economics 28 2007
The Price of Innovation: New Estimates of Drug Development Costs J. A. DiMasi, et al. Journal of Health Economics 22 2003
The R&D Process¶
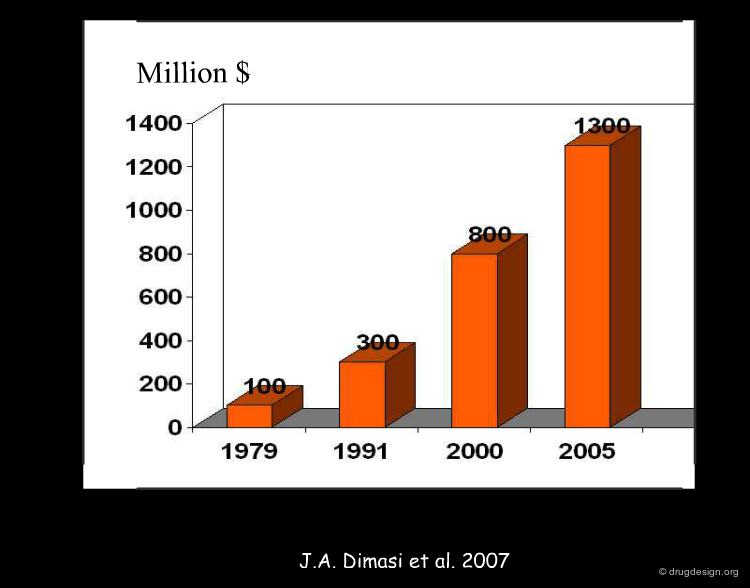
In the figure below is shown the entire R&D process necessary for discovering and commercializing a new drug. Out of 10,000 molecules explored, one may reach the market. The process requires about 10-15 years and cost about $1.3 billions dollars to the pharmaceutical company.
Innovation of Drug Discovery (1997-2007)¶
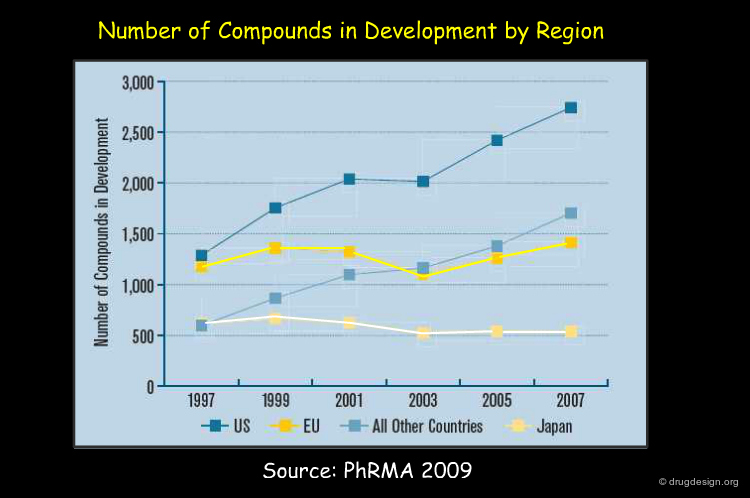
The graph below illustrates that innovation in drug discovery progressed in the US at substantial speed in the decade 1997-2007, whereas it did not progress so much in the EU and in Japan during the same period.
Improvements in Life Expectancy¶
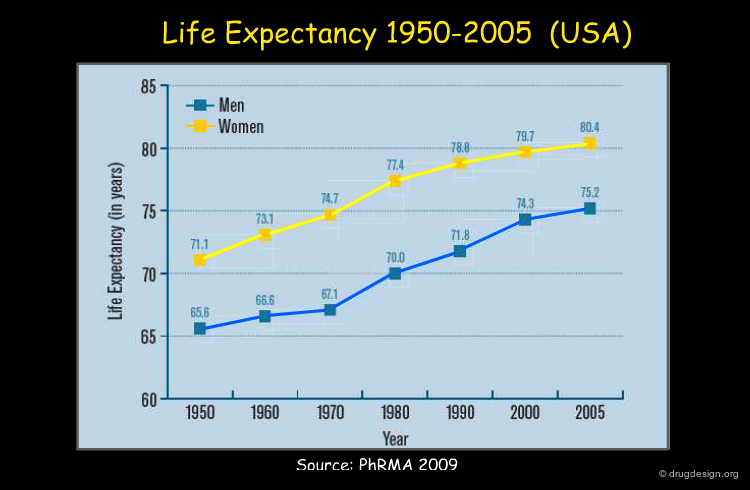
The progress made in improving life expectancy are visualized in the diagram below. Seven years were gained within a period of 30 years. Public health policies but also drug discovery achievements were important factors that contributed to such results. The trend is expected to continue.
Drug Discovery in the Industry¶
The following list gives an idea of the therapeutic agents searched in the pharmaceutical companies. Drug discovery is a major determinant in the successful development in all these areas.
Success of Drug Discovery (in 2008)¶
Below are shown the top 10 prescription drugs sold in the US market in 2008. These sales illustrate concrete results obtained by the drug discovery programs developed in the pharmaceutical industry.
60 Years of Drug Discovery Achievements¶
We present below a table illustrating some major discovery achievements that were made made in the course of 60 years of R&D, and starting from the discovery of sulfamides in 1936.
Concluding Remarks¶
Drug discovery has contributed to move an old apothecary practice to advanced computerized rational drug design technologies. The field has become highly competitive and created one of the most knowledge intensive industry. This also contributed to transform Health into a Product as a source of consumer goods for the benefit of the patients, the health organizations and the pharmaceutical industry. With the genomics era, drug discovery is now delivering intelligent personalized medicines of great value.
Media
Copyright © 2024 drugdesign.org