Bioisosterism¶
Info
The concept of bioisosterism is presented and put in perspective from the early ideas of Langmuir to the modern bioinformatics era. Because of its fuzziness the concept can be used in medicinal chemistry for many purposes and this is extensively presented and illustrated in this chapter. Patent issues associated with bioisosterism are discussed; programs and databases that have been developed for bioisoteric replacement are reviewed.
Number of Pages: 133 (±3 hours read)
Last Modified: February 2007
Prerequisites: None
Introduction¶
What is Bioisosterism?¶
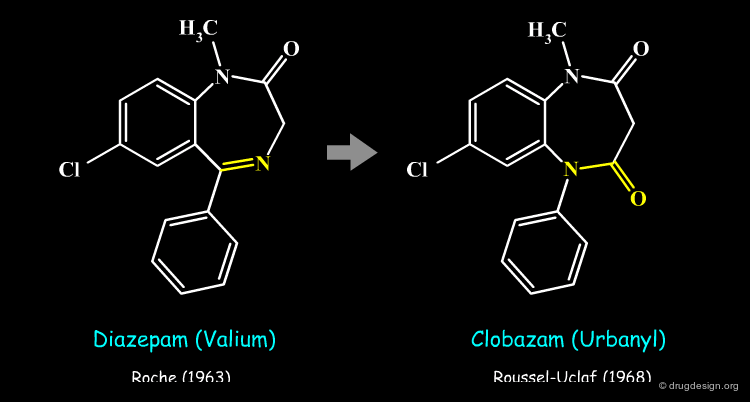
The principle that similar molecules tend to have similar properties has been around since the early days of medicinal chemistry. Bioisosterism, a concept based on this principle, makes it possible to replace fragments of a compound by groups having similar properties. The modified analog is then tested for interesting properties. The example below illustrates two bioisosteric anxiolitic drugs: Diazepam and Clobazam. Clobazam was found to be more effective than diazepam in diminishing anxiety.
articles
Isosterism and molecular modification in drug design Thornber C.W. Chem.Soc.Rev 8 1979
Bioisosterism: A Rational Approach in Drug Design Patani GA, LaVoie EJ. Chem Rev 96(8) 1996
The use of bioisosteric groups in lead optimization Olesen PH Curr Opin Drug Discov Devel 4(4) 2001
Bioisosterism: a useful strategy for molecular modification and drug design Lima LM, Barreiro EJ. Curr Med Chem 12(1) 2005
Chemical similarity and biological activities Kubinyi H J Braz Chem Soc 13(6) 2002
Bioisosterism and Molecular Diversity Robert D. Clark, Allan M. Ferguson, and Richard D. Cramer Perspectives in Drug Discovery and Design 9-10-11 1998
Bioisosterism as a molecular diversity descriptor: steric fields of single "topomeric" conformers Cramer RD, Clark RD, Patterson DE, Ferguson AM. J Med Chem 39(16) 1996
Study of an anxiolytic, clobazam, in otorhinolaryngology in psychosomatic pharyngeal manifestations (in French) Freche, C. Semaine des Hopitaux; Therapeutique 51 (4) 1975
book
Burger A. Prog. Drug. Res. Vol. 37 Birkhauser Publ. 1991
Janos Fischer and Robin C. Ganellin Analogue-based Drug Discovery Wiley-VCH 2006
History of the Concept of Bioisosterism¶
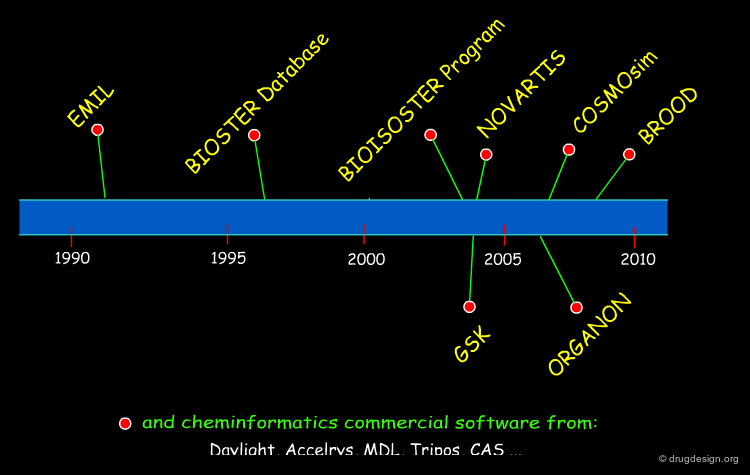
Over the years the concept of bioisosterism has progressively evolved. In 1919 Langmuir introduced the concept of isosterism that only took physicochemical properties into account. Later the biological dimension was included that turned the original isosterism into bioisosterism. Recent progress has given a new dimension to bioisosterism and has made it an integral part of cheminformatics today. The key phases in the development of bioisosterism appear in the following diagram and are presented in the following pages.
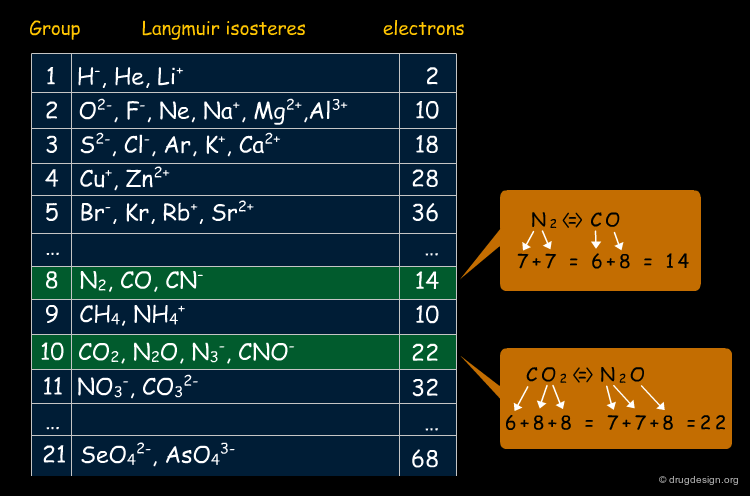
Langmuir (1919): Comolecules and Isosteres¶
Langmuir introduced the concept of "isosteres" (or "comolecules") by observing that molecules such as N2 and CO, CO2 and N2O had similar physical properties. He suggested that molecules or groups of atoms that have the same number of electrons may possess similar physicochemical properties. He was able to identify 21 groups of isosteres some of which are shown below.
articles
Isomorphism, isosterism and covalence Langmuir, I. J.Am.Chem.Soc 41 1919
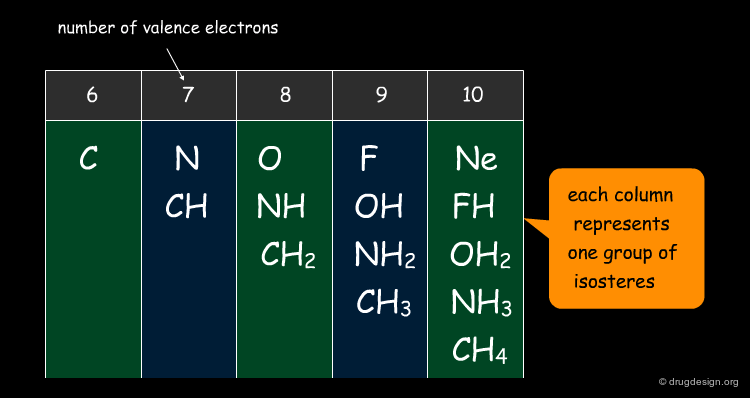
Grimm (1925)¶
While studying non-metallic hydrides, Grimm defined his "hydride displacement law" to describe structural similarities between groups of atoms that combine with hydrogen atoms. Each vertical column of the table below represents a group of isosteres: they have the same number of valence electrons.
articles
Structure and Size of the Non-metallic Hydrides Grimm, H. G. Z. Electrochem 31 1925
**On the Systematic Arrangement of Chemical
Compounds from the Perspective of Research on Atomic Composition; and on Some Challenges in Experimental Chemistry** Grimm, H. G. Naturwissenschaften 17 1929
Erlenmeyer (1932)¶
Erlenmeyer extended Grimm's classification and defined isosteres as atoms, ions, and molecules in which the peripheral layers of electrons are identical. In the following table isosteres are grouped according to the number of peripheral electrons. Each vertical column represents a group of isosteres.
articles
On Pseudoatoms Erlenmeyer, H.; Leo, M. Helv. Chim. Acta 15 1932
Friedman (1951): Concept of Bioisosteres¶
Friedman introduced the term "bioisosterism" which defined bioisosteres as a group of atoms or molecules that are structurally similar and show the same type of biological activity. Similar compounds with opposite biological activities (e.g. agonist/antagonist) are also considered bioisosteres.
book
Friedman, H.L. National Research Council Publication No 206. National Academy of Sciences 1951
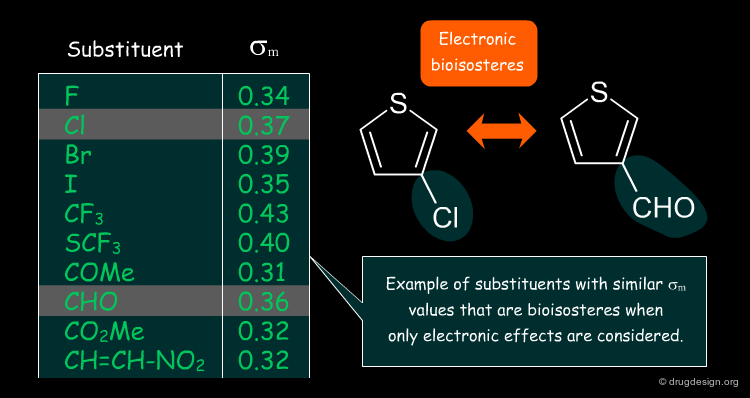
Thornber (1979)¶
Thornber further characterized the field of bioisosteres to include groups or molecules that have similar physicochemical properties or similar biological effects. He suggested guiding bioisosteric replacements by comparing the Hansch's π values, Hammett's Σ constants and Taft's Es steric parameters of substituents as a measure of their physicochemical properties.
articles
Isosterism and molecular modification in drug design Thornber C.W. Chem.Soc.Rev 8 1979
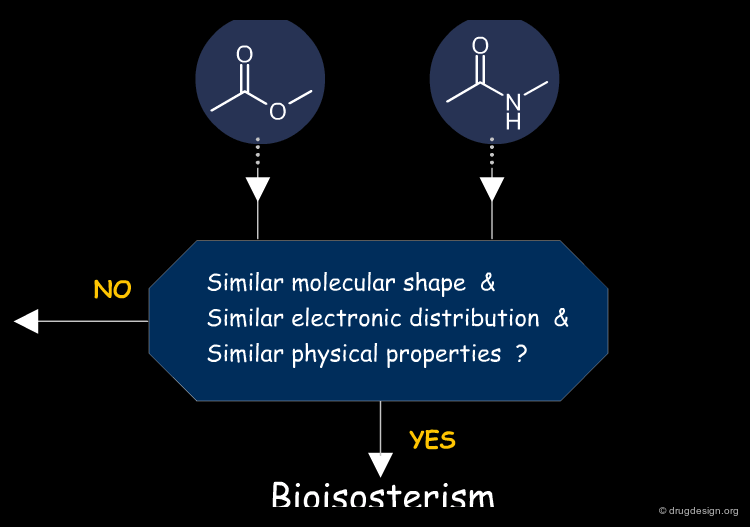
Burger (1991)¶
Burger modified somewhat Friedman's definition of bioisosteres defining them as molecules or groups of atoms that possess similar molecular shapes and volumes, approximately the same distribution of electrons, and exhibit similar physical properties. This definition proved to be of great utility to the medicinal chemist because of - rather than despite - its fuzziness.
book
Burger A. Prog. Drug. Res. Vol. 37 Birkhauser Publ. 1991
Cheminformatics Era (1993)¶
Bioisosterism has recently attracted renewed interest because of the large-scale application of computers in drug design, which have opened up the era cheminformatics and enabled molecular similarity to be considered in a high throughput quantitative manner. This is presented in great detail in the chapter on "molecular similarity" in the cheminformatics volume.
Remark on Stereochemical Aspects¶
Stereochemistry can be viewed as a special case of bioisosterism but this feature is beyond the scope of the present chapter. Chiral-switch will be however briefly mentioned, in the section on patent issues.
articles
Intellectual property and chirality of drugs Israel Agranat and Hava Caner Drug Discovery Today 4 (7) 1999
Putting chirality to work: the strategy of chiral switches Agranat I, Caner H, Caldwell J. Nat Rev Drug Discov 1 (10) 2002
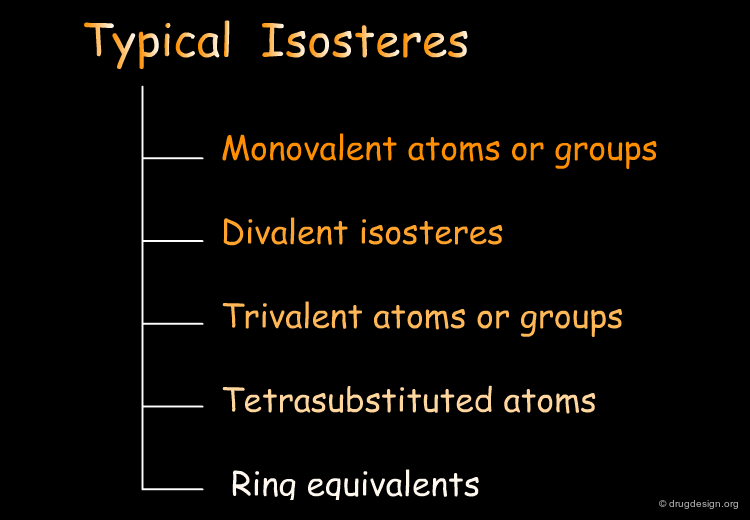
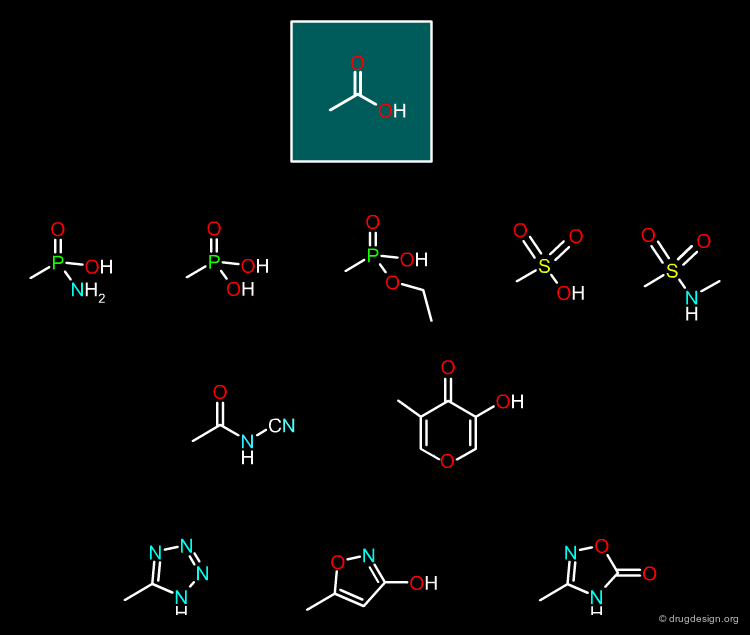
Typical Isosteres¶
Classification of Typical Isosteres¶
Typical bioisosteres obey Burger's steric and electronic definition and will be presented in the following pages according to the classification indicated below.
Monovalent Atoms or Groups¶
Some examples of monovalent isosteres are illustrated in the following figure.
book
Richard Silverman The organic chemistry of drug design and drug action Elsevier, Academic Press 2004
Divalent Isosteres¶
Some examples of divalent bioisosteres are shown in the following view.
book
Richard Silverman The organic chemistry of drug design and drug action Elsevier, Academic Press 2004
Trivalent Atoms or Groups¶
Some examples of trivalent bioisosteres are shown in the following figure.
book
Richard Silverman The organic chemistry of drug design and drug action Elsevier, Academic Press 2004
Tetrasubstituted Atoms¶
Some tetrasubstituted bioisosteres are indicated in the following view.
book
Richard Silverman The organic chemistry of drug design and drug action Elsevier, Academic Press 2004
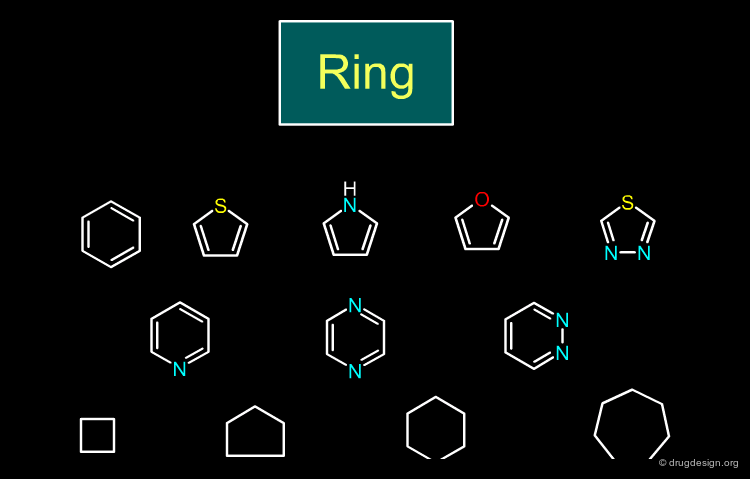
Ring Equivalents¶
The following view displays examples of equivalent ring bioisosteres.
Medicinal Chemistry Use¶
A Simple Concept for Many Applications¶
When the target molecule has to be modified, bioisosterism can help guide the process, and medicinal chemists use it extensively as a tool in their queries for new molecules. As shown in the list below, the needs of chemists can be very different. However, due to its simplicity, bioisosterism provides a simple and creative way to address all these in a constructive manner as is illustrated in the following pages.
articles
Design and SAR of novel potassium channel openers targeted for urge urinary incontinence. 1. N-Cyanoguanidine bioisosteres possessing in vivo bladder selectivity Butera JA, Antane MM, Antane SA, Argentieri TM, Freeden C, Graceffa RF, Hirth BH, Jenkins D, Lennox JR, Matelan E, Norton NW, Quagliato D, Sheldon JH, Spinelli W, Warga D, Wojdan A, Woods M. J Med Chem 43 (6) 2000
Adapt Chemical Structures to Feasible Syntheses¶
The synthetic scheme of the chemical target is the first issue addressed by the chemist. If the synthesis proves to be complicated, he envisages changing the initial target in order to obtain molecules that are easy to prepare. In the example below, the initial target has been changed, because of the commercial availability of a simple starting material.
articles
Syntheses of novel heterocycles as anticancer agents Prem M. S. Chauhan, Cristina J. A. Martins and David C. Horwell Bioorganic and Medicinal Chemistry 13 2005
Change the Type of Biological Activity¶
In some cases the medicinal chemist does not know exactly what he is looking for. His synthetic program is then based on the modification of the structure of an active compound, with the hope of finding new analogs with unexpected biological profiles. In the following pages we present three examples to illustrate how bioisosterism can guide this type of approach.
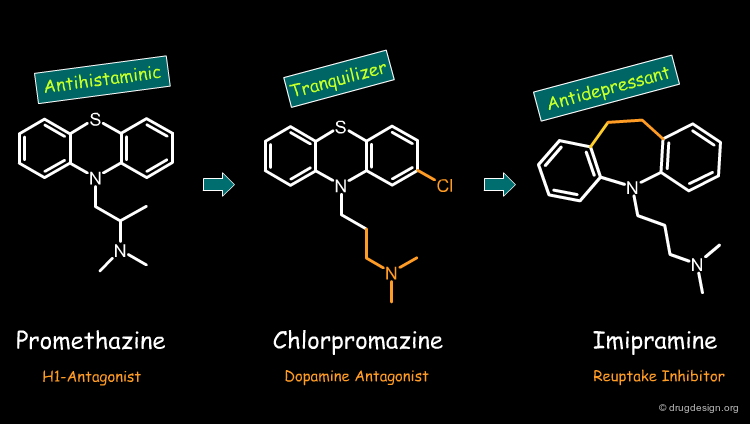
Example 1: Tricyclic Structures¶
Simple bioisosteric modifications of the phenothiazine structure proved to be a rich source of useful drugs having completely different modes of action. Starting with antihistaminic drugs such as promethazine, it has been possible to discover bioisostere tranquillizers such as chlorpromazine. Further bioisoteric modifications led to the discovery of antidepressant drugs such as imipramine. Note the different mode of action of these drugs.
book
The Merck Index Published by: Merck and Co. Whitehouse Station, NJ 1996
Example 2: Angiotensin-II Receptor Ligands¶
Simple bioisosteric replacements in a drug can lead to entirely different modes of action. In the example illustrated below, bioisosterism was employed to slightly modify the structure of an antagonist (L-162,389) and discover a useful agonist (L-162,782).
articles
Dual agonistic and antagonistic property of nonpeptide angiotensin AT1 ligands: susceptibility to receptor mutations Perlman S, Costa-Neto CM, Miyakawa AA, Schambye HT, Hjorth SA, Paiva AC, Rivero RA, Greenlee WJ, Schwartz TW. Mol Pharmacol 51 (2) 1997
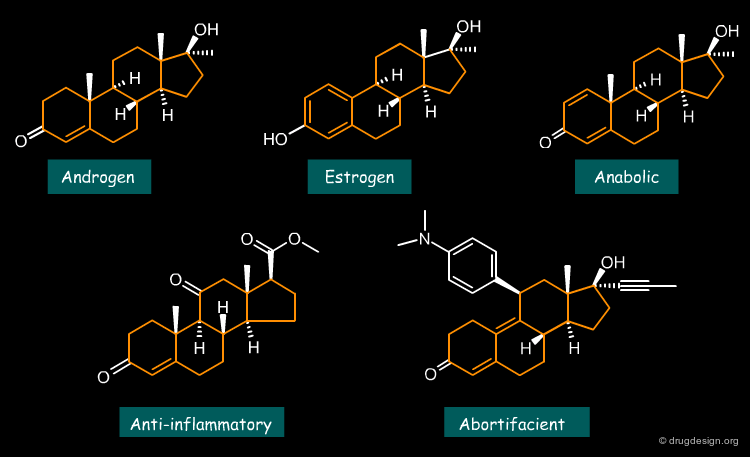
Example 3: Steroid Analogs¶
The steroid nucleus is full of diverse biological properties. All the analogs shown below can be considered as bioisosteres however, each of them has an entirely different mode of action acting as an androgen, estrogen, anti-inflammatory, anabolic or abortifacient drug.
book
The Merck Index Published by: Merck and Co. Whitehouse Station, NJ 1996
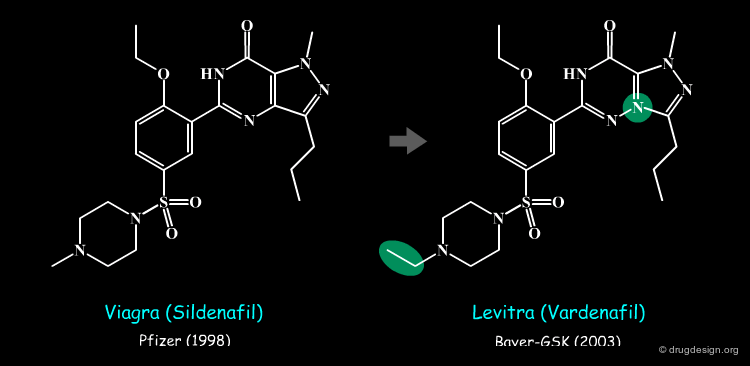
Achieve Patentability¶
Using bioisosterism can be an effective way to circumvent a patent owned by a competitor. Levitra (vardenafil) is an example of bioisosteric replacement that enabled the Bayer/GSK team to achieve patentability to compete with the Pfizer viagra (sildenafil) drug. The FDA approval dates (for male erectile dysfunctions) are indicated in parentheses.
articles
Vardenafil Bayer Yakuhin Sommer F, Engelmann U. Curr Opin Investig Drugs 3(4) 2002
Comparison of phosphodiesterase type 5 (PDE5) inhibitors Wright PJ. Int J Clin Pract
. 2006
Mimic an Endogenous Ligand¶
Bioisosterism has proven a useful concept for molecular mimicry. In the example below, the carboxylic group of glutamic acid was replaced by a 3-hydroxy-5-methyl-4-isoxazole bioisostere, leading to a molecule that is a potent agonist of the central glutamic acid receptor.
articles
Novel 1-hydroxyazole bioisosteres of glutamic acid. Synthesis, protolytic properties, and pharmacology Stensbol TB, Uhlmann P, Morel S, Eriksen BL, Felding J, Kromann H, Hermit MB, Greenwood JR, Brauner-Osborne H, Madsen U, Junager F, Krogsgaard-Larsen P, Begtrup M, Vedso P. J Med Chem 45(1) 2002
Improve Potency¶
In the example below, simple bioisosteric replacements served to improve the potency of the initial kynurenic acid lead (IC50 = 41 µM) by a factor of 1000 (IC50 = 32 nM for compound L-683,334).
articles
7-Chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the N-methyl-D-aspartate receptor complex Kemp JA, Foster AC, Leeson PD, Priestley T, Tridgett R, Iversen LL and Woodruff GN Proc. Natl. Acad. Sci. U.S.A 85 1988
book
Lesson PD, Carling RW, Kulagowski JJ, Mawer IM, Moore KW, Moseley AM, Rowley M, Smith JD, Stevenson GI, Williams BJ, Baker R, Foster AC, Kemp JA and Tricklebank MD Prespectives in Medicinal Chemistry Verlag Helvetica Chimica Acra, Basel 1993
Improve Selectivity¶
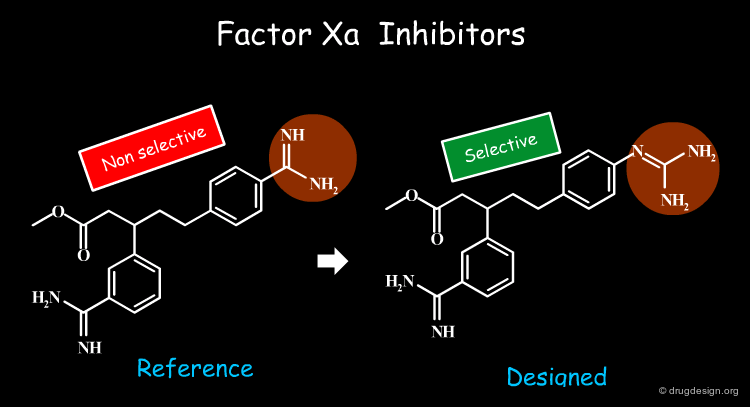
When the structure of the target protein is not known, selectivity cannot be achieved in a rational structure-based way. Bioisosterism provides a good alternative for the systematic alteration of the structure of an initial lead in order to achieve selectivity. The example illustrated here concerns Factor Xa inhibitors for which the selectivity was improved by a factor of 10.
articles
The Geometry of the Reactive Site and of the Peptide Groups in Trypsin, Trypsinogen and its Complexes with Inhibitors Marquart M, Walter J, Deisenhofer J, Bode W and Huber R Acta Crystallographica Sect. B 39 1983
Reduce Side Effects¶
The Fluoxetine (Prosac) project aimed at the discovery of an antidepressant drug acting by inhibiting the reuptake of serotonin (5-HT). The initial lead had the desired biological action with, however, considerable side effects. It was hypothesized that they were due to action on the reuptake of norepinephrine (NE). The introduction of a trifluoromethyl group in the para position of the phenol moiety led to a compound which had the desired antidepressant action with significantly fewer side effects.
articles
Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication Wong DT Life Sciences 57 (5) 1995
Reduce Toxicity¶
Colchicine is a potent mitotic inhibitor that could not be widely used as a drug because of its high toxicity. The bioisostere analog shown below was found to be less toxic.
articles
Synthesis and biological effects of novel thiocolchicines. 3. Evaluation of N-acyldeacetylthiocolchicines, N-(alkoxycarbonyl) deacetylthiocolchicines, and O-ethyldemethylthiocolchicines. New synthesis of thiodemecolcine and antileukemic effects of 2-demethyl- and 3-demethylthiocolchicine Peter Kerkes, Padam N. Sharma, Arnold Brossi, Colin F. Chignell, and Frank R. Quinn J Med Chem 28(9) . 1985
Improve Bioavailability¶
Poor bioavailability is very often associated with poor water solubility. In the example shown below (thrombin inhibitors) a simple bioisosteric replacement substantially improved the water solubility of the initial lead and eventually led to an oral drug.
articles
Discovery of LB30057, a benzamidrazone-based selective oral thrombin inhibitor YS Oh Biorg. Med. Chem. Lett 8 (6) 1998
Exploit Metabolism¶
Quinine is a drug used to treat malaria and it was observed that the compound is readily oxidized (by cytochrome P450) at the 2'-position. The introduction of a substituent in position 2' enabled researchers to obtain simpler drugs with a longer half-life. Mefloquine is one example of such drugs.
articles
Studies of the disposition and metabolism of mefloquine HCl (WR 142,490), a quinolinemethanol antimalarial, in the rat. Limited studies with an analog, WR 30,090 Mu JY, Israili ZH, Dayton PG. Drug Metab Dispos 3 1975
Modify pKa¶
Cocaine is a potent brain stimulant that binds to the dopamine transporter DAT. The molecule has an IC50 of 173 nM, and it has been hypothesized that the increase of the basicity of the nitrogen atom may lead to more potent structures. Indeed, several bioisosteres of cocaine were designed whose potency was found to be correlated with a high pKa (nitrogen atom more basic). For example a very basic compound was found (pKa = 11.99) with an IC50 of 0.59 nM.
articles
Conformational, Aqueous Solvation, and pKa Contributions to the Binding and Activity of Cocaine, WIN 35 065-2, and the WIN Vinyl Analog Yang B, Wright J, Eldefrawi ME, Pou S and MacKerell AD HJ. Am. Chem. Soc 116 1994
Chemistry, Design, and Structure-Activity Relationship of Cocaine Antagonists Singh S Chem. Rev 100 2000
Increase Chemical Stability¶
Following-up on the piperidine-based renin inhibitor discovered by Roche (reference), Pfizer designed the piperazine structure shown below (design-1). The compound had the desired biological activities but was chemically unstable. The ketopiperazine analog (design-2) was suggested to circumvent this problem and indeed proved to be stable. At each step of the project the design was guided by bioisoteric considerations that finally led to simpler inhibitors.
articles
Equipotent activity in both enantiomers of a series of ketopiperazine-based renin inhibitors Noel A. Powell, Emma H. Clay, Daniel D. Holsworth, John W. Bryant, Michael J. Ryan, Mehran Jalaie, Erli Zhang and Jeremy J. Edmunds Bioorganic and Medicinal Chemistry Letters 15 2005
The discovery and preparation of disubstituted novel amino-aryl-piperidine-based renin inhibitors Wayne L. Cody,Daniel D. Holsworth, Noel A. Powell, Mehran Jalaie, Erli Zhang, Wei Wang, Brian Samas, John Bryant, Robert Ostroski, Michael J. Ryand and Jeremy J. Edmunds Bioorganic and Medicinal Chemistry 13 2005
Discovery of novel non-peptidic ketopiperazine-based renin inhibitors Daniel D. Holsworth, Noel A. Powell, Dennis M. Downing, Cuiman Cai,Wayne L. Cody, J. Michael Ryan, Robert Ostroski, Mehran Jalaie, John W. Bryantb and Jeremy J. Edmunds Bioorganic and Medicinal Chemistry 13 2005
Ketopiperazine-based renin inhibitors: Optimization of the C ring Daniel D. Holsworth, Cuiman Cai, Xue-Min Cheng, Wayne L. Cody, Dennis M. Downing,a Noe Erasga,a Chitase Lee,a Noel A. Powell,a Jeremy J. Edmunds,a Michael Stier, Mehran Jalaie, Erli Zhang, Pat McConnell, Michael J. Ryan, John Bryant, Tingsheng Li, Aparna Kasani, Eric Hall, Rajendra Subedi, Mohammad Rahim and Samarendra Maiti Bioorganic and Medicinal Chemistry Letters 16 2006
Combinatorial Chemistry¶
In combinatorial chemistry, the bioisosterism concept can be exploited for building libraries in a systematic manner. In the example below a library was prepared by focusing on the benzonitrile pharmacophore of the androgen receptor antagonist compound GSK-7721. Bioisoterism was considered for the fluoroaryl fragment 1, whereas structural diversity was left variable for the amine moiety 2.
articles
Design and Synthesis of an Array of Selective Androgen Receptor Modulators Ryan P. Trump, Jean-Baptiste E. Blanc, Eugene L. Stewart, Peter J. Brown, Matilde Caivano, David W. Gray, William J. Hoekstra, Timothy M. Willson, Bajin Han, and Philip Turnbull Journal of Combinatorial Chemistry ASAP Article 10.1021/cc060096e 2006
Examples of Natural Bioisosteres¶
Bioisosteres in Nature¶
Natural molecules can be highly diverse and can show very subtle similarities; many bioisosteres exist in nature. In the following pages we illustrate this feature by showing some natural bioisosteres that can be found in amino-acids, nucleotides, sugars, lipids, steroids, carbohydrates, catecholamines and β-lactam antibiotics.
Aminoacids¶
Phenylalanine, tyrosine, tryptophan and histidine are examples of amino-acid bioisosteres that are found in proteins. The phenyl ring of phenylalanine becomes phenol in tyrosine, indole in tryptophan and imidazole in histidine.
book
The Merck Index Published by: Merck and Co. Whitehouse Station, NJ 1996
Nucleotides¶
Uracil and thymine, adenine and guanine are two examples of nucleotide bioisosteres that are found in nucleic acids, with very closely related structures.
book
The Merck Index Published by: Merck and Co. Whitehouse Station, NJ 1996
Sugars¶
α-D-ribose and 2-deoxy-α-D-ribose structures illustrate sugar bioisosteres that are found in nature. There is one more hydroxyl group in the structure of α-D-ribose.
book
The Merck Index Published by: Merck and Co. Whitehouse Station, NJ 1996
Lipids¶
Palmitic acid and palmitoleic acid, arachidic acid and arachidonic acid are two examples of natural bioisosteres that are found in lipids. The number of carbon atoms is the same for each pair of molecules but the structures differ by the number of double bonds in the chain.
book
The Merck Index Published by: Merck and Co. Whitehouse Station, NJ 1996
Steroid Hormones¶
Testosterone and estradiol structures illustrate steroid bioisosteres that are found in nature. The A-ring of testosterone is a cyclohexenone fragment, whereas in estradiol it consists of a phenol moiety.
book
The Merck Index Published by: Merck and Co. Whitehouse Station, NJ 1996
Carbohydrates¶
The structures of D-glucose and D-mannose illustrate bioisosteres that are found in carbohydrates. The two compounds differ by the stereochemistry of one hydroxyl group.
book
The Merck Index Published by: Merck and Co. Whitehouse Station, NJ 1996
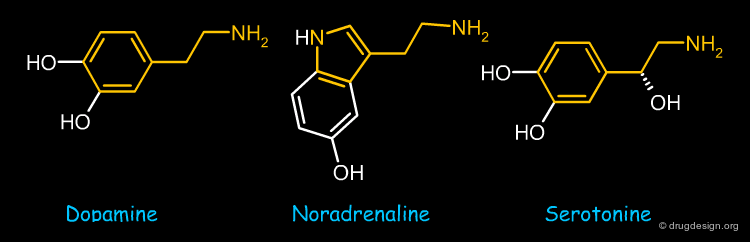
Catecholamines¶
Dopamine, noradrenaline and serotonine are bioisostere catecholamine structures that are found in the brain. They all have an arylethylamine moiety in common.
book
The Merck Index Published by: Merck and Co. Whitehouse Station, NJ 1996
Penicillins and Cephalosporins¶
Penicillins and cephalosporins are bioisotere antibiotic molecules that are found in nature. In penicillins the β-lactam ring is fused to a 5-membered ring, whereas in cephalosporins it is fused to a 6-membered ring. Δ-3 and Δ-2 cephalosporins differ by the position of the double bond in the 6-membered ring.
book
The Merck Index Published by: Merck and Co. Whitehouse Station, NJ 1996
Dictionary of Bioisosteres¶
Dictionary of Bioisosteric Replacements¶
The following section presents bioisosteric replacements for some chemical groups that are listed here in alphabetical order.
articles
Isosterism and molecular modification in drug design Thornber C.W. Chem.Soc.Rev 8 1979
book
Burger A. Prog. Drug. Res. Vol. 37 Birkhauser Publ. 1991
Richard Silverman The organic chemistry of drug design and drug action Elsevier, Academic Press 2004
Allyl¶
The cyclopropylmethylene fragment is a bioisostere of the allyl moiety.
articles
4-Amino-2-alkyl-butyramides as small molecule CCR2 antagonists with favorable pharmacokinetic properties Gabor Butora, Gregori J. Morriello, Shankaran Kothandaraman, Deodialsingh Guiadeen, Alexander Pasternak, William H. Parsons, Malcolm MacCoss, Pasquale P. Vicario, Margaret A. Cascieri and Lihu Yang Bioorganic and Medicinal Chemistry Letters 16 2006
** **
Amide¶
There are many bioisosteres of the amide group, which are indicated in the figure below.
book
Richard Silverman The organic chemistry of drug design and drug action Elsevier, Academic Press 2004
Amino-Acids¶
Many criteria can be used to replace an amino-acid with another one. Similarities of properties such as hydrophobicity, aromaticity, size, electronic features etc... can be considered alone or simultaneously. The following diagram helps guide the choice of possible bioisosteric replacements.
book
Chemistry and Biochemistry of Amino Acids Chapman and Hall 1985
Greenstein JP, and Winitz M Chemistry of the Amino Acids John Wiley & Sons 1961
Azomethine¶
In the figure below is shown a possible bioisosteric replacement for the azomethine group.
articles
Isosterism and molecular modification in drug design Thornber C.W. Chem.Soc.Rev 8 1979
book
Richard Silverman The organic chemistry of drug design and drug action Elsevier, Academic Press 2004
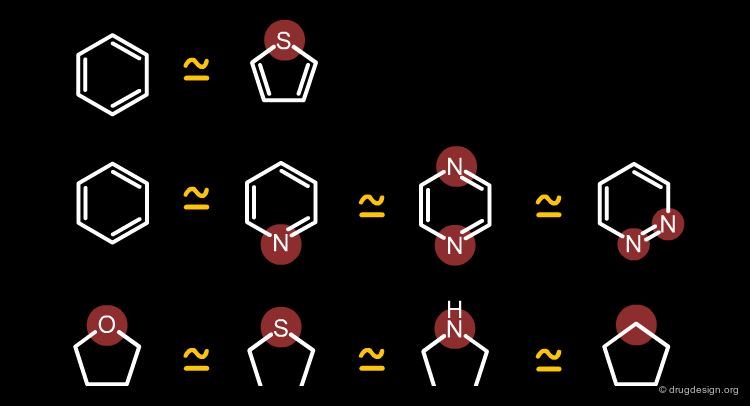
Benzene¶
The benzene moiety can be replaced by various 6-membered or 5-membered heterocyclic structures. The bioisoterism between benzene and thiophene has long been recognized; the sulfur atom of thiophene is considered as equivalent (geometrically and electronically) to one double bond of the benzene ring.
book
Richard Silverman The organic chemistry of drug design and drug action Elsevier, Academic Press 2004
Carbonyl¶
Possible bioisosteres of the carbonyl group are indicated in the figure below.
articles
Isosterism and molecular modification in drug design Thornber C.W. Chem.Soc.Rev 8 1979
book
Richard Silverman The organic chemistry of drug design and drug action Elsevier, Academic Press 2004
Carboxylic Acid¶
There are many possible bioisosteric replacements for the carboxylic acid group, as visualized in the figure below.
articles
Isosterism and molecular modification in drug design Thornber C.W. Chem.Soc.Rev 8 1979
book
Richard Silverman The organic chemistry of drug design and drug action Elsevier, Academic Press 2004
Catechol¶
The catechol group can be replaced by several bioisosteric equivalent groups, as shown in the figure below.
articles
Isosterism and molecular modification in drug design Thornber C.W. Chem.Soc.Rev 8 1979
book
Richard Silverman The organic chemistry of drug design and drug action Elsevier, Academic Press 2004
Ester¶
The ester group can be replaced by a 1,2,4 oxadiazole or by an amide group, as shown in the figure below.
book
Richard Silverman The organic chemistry of drug design and drug action Elsevier, Academic Press 2004
Halogen¶
Possible bioisosteres of a halogen atom are indicated in the figure below.
articles
Isosterism and molecular modification in drug design Thornber C.W. Chem.Soc.Rev 8 1979
book
Richard Silverman The organic chemistry of drug design and drug action Elsevier, Academic Press 2004
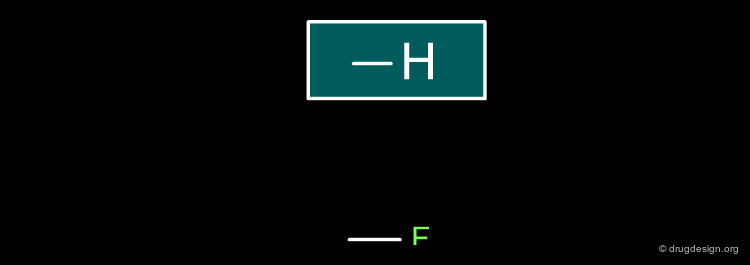
Hydrogen¶
A hydrogen atom can be replaced by a fluorine atom.
book
Richard Silverman The organic chemistry of drug design and drug action Elsevier, Academic Press 2004
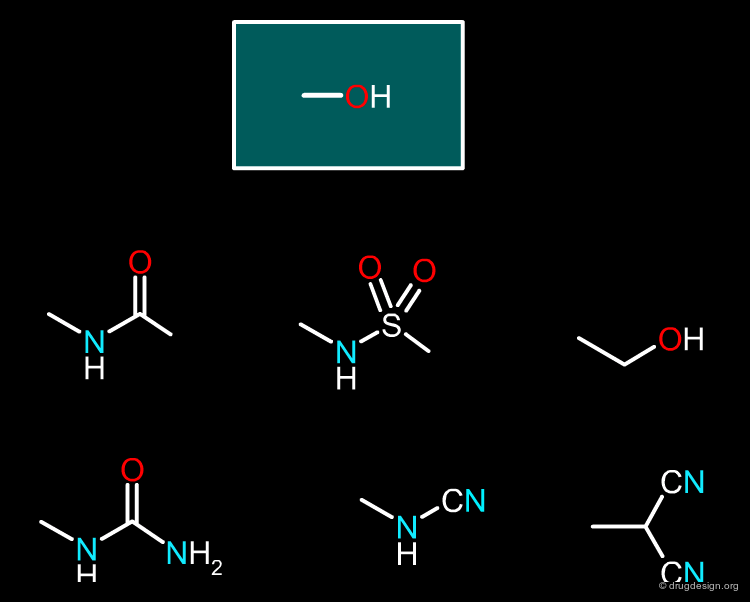
Hydroxyl¶
Many isosteric replacements were found to be possible for the hydroxyl group, as shown in the figure below.
articles
Isosterism and molecular modification in drug design Thornber C.W. Chem.Soc.Rev 8 1979
book
Richard Silverman The organic chemistry of drug design and drug action Elsevier, Academic Press 2004
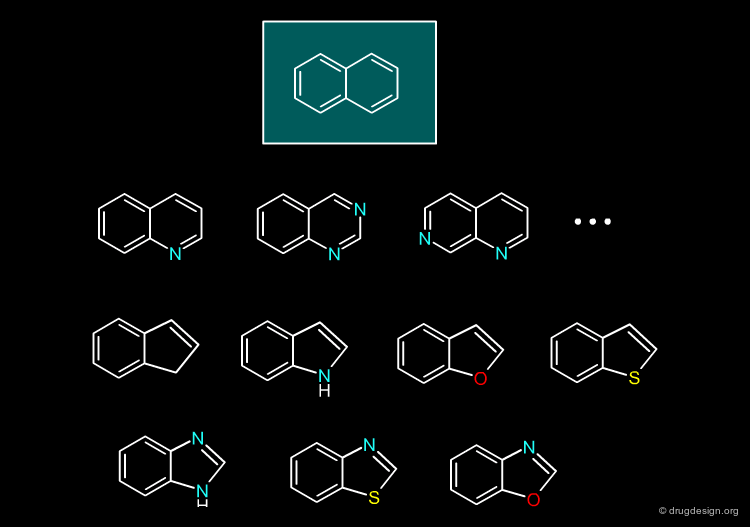
Indole¶
The indole moiety can be replaced by a benzimidazole, a benzoxazole or an indazole bioisostere.
articles
Bioisosterism: a useful strategy for molecular modification and drug design Lima LM, Barreiro EJ. Curr Med Chem 12(1) 2005
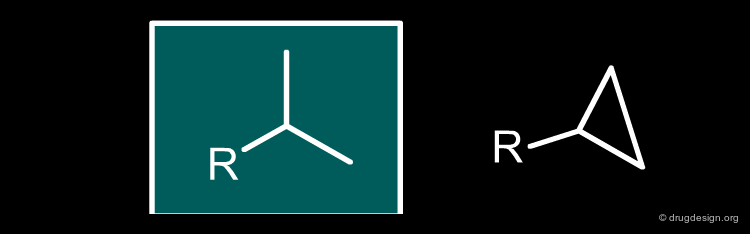
Isopropyl¶
An isopropyl fragment can simply be replaced by the corresponding cyclopropyl bioisostere.
articles
3-Amino-1-alkyl-cyclopentane carboxamides as small molecule antagonists of the human and murine CC chemokine receptor Gabor Butora, Richard X. Jiao, William H. Parsons, Pasquale P. Vicario, Jin Hong, Julia M. Ayala, Margaret A. Cascieri, and Lihu Yang 232nd ACS Meeting San Francisco
September 2006
Synthesis and Biological Activity of Some Transition-state Inhibitors of Human Renin Buhlmayer P, Caselli A, Fuhrer W, Goschke R, Rasetti V, Rueger H, Stanton JL, Criscione L and Wood JM J. Med. Chem 31 1988
Remikiren (Ro 42-5892)-an orally active renin inhibitor in essential hypertension. Effects on blood pressure and the renin-angiotensin-aldosterone system Himmelmann A, Bergbrant A, Svensson A, Hansson L, Aurell M. Am. J. Hypertens 9(6) 1996
Naphthalene¶
A naphthalene fragment can be replaced by many different structural moieties. Carbon atoms can be replaced by nitrogens and the six-membered rings can be replaced by aromatic five-membered rings.
articles
Isosterism and molecular modification in drug design Thornber C.W. Chem.Soc.Rev 8 1979
A comparison of intravenous pindolol and propranolol in normal man Hicks DC, Arbab AG, Turner P, Hills M. J Clin Pharmacol New Drugs 12(5) 1972
Piperidine-renin inhibitors compounds with improved physicochemical properties Guller R, Binggeli A, Breu V, Bur D, Fischli W, Hirth G, Jenny C, Kansy M, Montavon F, Muller M, Oefner C, Stadler H, Vieira E, Wilhelm M, Wostl W, Marki HP. Bioorg Med Chem Lett 9(10) 1999
Epidermal growth factor receptor tyrosine kinase: Structure-activity relationships and antitumour activity of novel quinazolines K. H. Gibson, W Grundy, A. A. Godfrey, J. R. Woodburn, S. E. Ashton, B. J. Curry, L Scarlett, A. J. Barker and D. S. Brown Bioorganic and Medicinal Chemistry Letters 7 (21) 1997
Synthesis and preliminary pharmacological results on new naphthalene derivatives as 5-HT[4] receptor ligands Diouf O; Depreux P; Chavatte P.and Poupaert J. H. Eur. J. Med. Chem 35 (7-8) 2000
Piperidine renin inhibitors: from leads to drug candidates Marki HP, Binggeli A, Bittner B, Bohner-Lang V, Breu V, Bur D, Coassolo PH, Clozel JP et al. Farmaco 56(1-2) 2001
Naphthalene combretastatin analogues: synthesis, cytotoxicity and antitubulin activity Medarde M, Maya AB, Perez-Melero C. Journal of Enzyme Inhibition and Medicinal Chemistry 19(6) 2004
Structure-activity relationships study of two series of leukotriene B[4] antagonists : Novel indolyl and naphthyl compounds substituted with a 2-[methyl(2-phenethyl)amino]-2-oxoethyl side chain Chan W. K. ; Huang F.-C. ; Morrissette M. M. ; Warus J. D. ; Moriarty K. J. ; Galemmo R. A. ; Dankulich W. D. ; Poli G. ; Sutherland C. A. J. Med. Chem 39 (19) 1996
** ** Sarah Chackal-Catoen, Yi Miao, W. David Wilson, Tanja Wenzler, Reto Brun and David W. Boykin Dicationic DNA-targeted antiprotozoal agents: Naphthalene replacement of benzimidazole 14 (22) 2006
Synthesis, anti-inflammatory, analgesic and kinase (CDK-1, CDK-5 and GSK-3) inhibition activity evaluation of benzimidazole/benzoxazole derivatives and some Schiff's bases Sham M. Sondhi, Nirupma Singh, Ashok Kumar, Olivier Lozach and Laurent Meijer Bioorganic and Medicinal Chemistry 14 (11) 2006
Structure-activity relationships for substrates and inhibitors of pineal 5-hydroxytryptamine-N-acetyltransferase: preliminary studies Shuren Shen, Beatrice Bremont, Isabelle Serraz, Jean Andrieux, Annie Poncet, Monique Mathe-Allainmat, Evelyne Chanut, Jean-Hugues Trouvin and Michel Langlois European Journal of Pharmacology 307 (2) 1996
Thiophene congeners of morpholine and allylamine type antifungals--syntheses and biological activities Bracher F, Papke T. Pharmazie 50 (8) 1995
Synthesis and Structure-Activity Relationships of Novel Naphthalenic and Bioisosteric Related Amidic Derivatives as Melatonin Receptor Ligands Patrick Depreux, Daniel Lesieur, Hamid At Mansour, Peter Morgan, H. Edward Howel1,l Pierre Renard, Daniel-Henri Caignard, Bruno Pfeiffer, Philippe Delagrange, Beatrice Guardiola, Said YOUS, Anne Demarque, Gerard Adam, and Jean Andrieux J.Med.Chem 37 1994
Synthesis and cytotoxic activities of analogues of thuriferic acid Madrigal B.; Puebla P.; Ramos A.C.; Pelaez R.; Gravalos D.G.; Caballero E.; Medarde M. Bioorganic and Medicinal Chemistry 10 (2) 2002
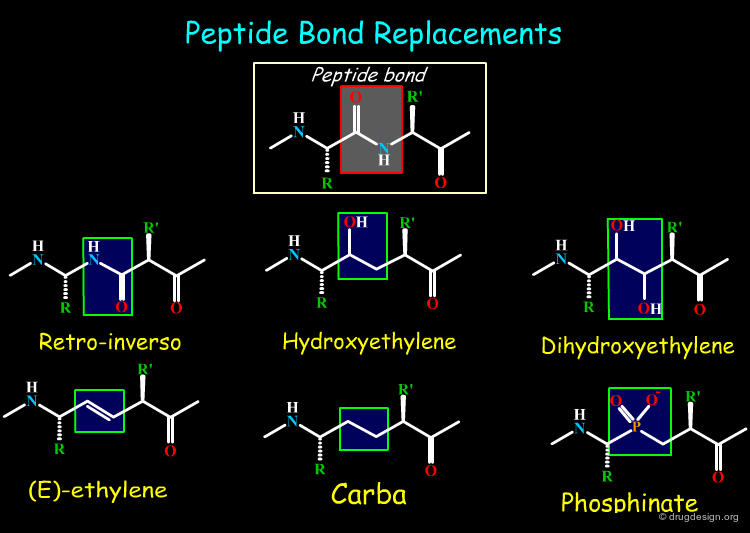
Peptide Surrogates¶
Many bioisosteric replacements of peptide molecules are possible. The replacements can address the N-H, the C=O, the peptide bond or the Cα atom. Click on the different buttons to see the corresponding series of bioisosteres.
articles
Azapeptides Gante J Synthesis 1989(6) 1989
Renin Inhibitors Greenlee WJ Med. Res. Rev 10 1990
Non-Standard Amino Acids in Peptide Design and Protein Engineering Balaram P Curr. Opin. Struct. Biol 2 1992
Control of Peptide Conformation by the Thorpe-Ingold Effect (C-alpha Tetrasubstitution) Toniolo C, Crisma M, Formaggio F and Peggion C Biopolymers 60 2001
Converting a Peptide into a Drug: Strategies to Improve Stability and Bioavailability Adessi C and Soto C Curr. Med. Chem 9 2002
Designing Non-peptide Peptidomimetics in the 21st Century: Inhibitors Targeting Conformational Ensembles Bursavich MG and Rich DH J. Med. Chem 45 2002
Peptidomimetics: Tailored Enzyme Inhibitors Gante J Angew. Chem. Int. Ed 33 1994
Peptidomimetics for Receptor Ligands Discovery, Development and Medical Prespectives Giannis A and Kotler T Angew. Chem. Int. Ed 32, 1993
Designing Peptide Mimetics Moore GJ Trends Pharmacol. Sci 15 1994
Molecular Modeling in the Design of Peptidomimetics and Peptide Surrogates Perez JJ, Corcho F and Llorens O Curr. Med. Chem 9 2002
Transformation of Peptides into Non-peptides. Synthesis of Computer-Generated Enzyme Inhibitors Rich DH, Bohacek RS, Xales NA, Glunz P and Ripka AS Chimia 51 1997
Structure-Based Inhibitors of HIV-1 Protease Wlodawer A and Erickson JW Annu. Rev. Biochem 62 1993
Peptidomimetic Design Ripka AS and Rich DH Curr. Opin. Chem. Biol 2 1998
Peptidomimetics Derived from Natural Products Wiley RA and Rich DH Med Res Rev 13 1993
Novel Renin Inhibitors Containing the Amino Acid Statine Boger J, Lohr NS, Ulm EH, Poe M, Blaine EH, Fanelli GM, Lin TY, Payne LS, Schorn TW, LaMont BI, Vassil TC, Stabilito II, Veber DF, Rich DH and Bopari AS Nature 303 1983
Potent New Inhibitors of Human Renin Szelke M, Leckie B, Hallett A, Jones DM, Sueiras J, Atrash B and Lever AF Nature 299 1982
Conformationally Restricted Peptides Through Short-Range Cyclizations Toniolo C Int. J. Pept. Protein Res 35 1990
Conformationally Constrained Amino Acids. Synthesis and Optical Resolution of 3-Substituted Proline Derivatives Chung JYL, Wasicak JT, Arnold WA, May CS, Nadzan AM, and Holladay MW J. Org. Chem 55 1990
Synthesis of Chiral Piperazin-2-ones as Model Peptidomimetics DiMaio J and Belleau B J. Chem. Soc. Perkin Trans. 1 9 1989
Bioactive Conformation of Luteinizing Hormone-Releasing Hormone: Evidence from a Conformationally Constrained Analog Freidinger RM, Veber DF, Perlow DS, Brooks JR and Saperstein R Science 210 1980
A New Approach to Receptor Ligand Design: Synthesis and Conformation of a New Class of Potent and Highly Selective Opioid Antagonists Utilizing Tetrahydroisoouinoline Carroxylic Acid Kazmierski W and Hruby VJ Tetrahedron 44 1988
A Molecular Constraint that Generates a cis Peptide Bond Sukumaran DK, Prorok M, and Lawrence DS J. Am. Chem. Soc 113 1991
Conformational Mimicry. 1. 1,5-Disubstituted Tetrazole Ring as a Surrogate for the Cis Amide Bond Zabrocki J, Smith GD, Dunbar JB Jr., Iijima H, and Marshall GR J. Am. Chem. Soc 110 1988
Emerging Approaches in the Molecular Design of Receptor-selective Peptide Ligands: Conformational, Topographical and Dynamic Considerations Hruby VJ, al-Obeidi F, and Kazmierski W Biochem. J 268 1990
Backbone Cyclization: A New Method for Conferring Conformational Constraint on Peptides Gilon C, Halle D, Chorev M, Selinger Z, and Byk G. Biopolymers 31 1991
Cyclization Strategies in Peptide Derived Drug Design Li P and Roller PP Curr. Top. Med. Chem 2 2002
Application of Ring-Closing Metathesis to the Synthesis of Rigidified Amino Acids and Peptides Miller SJ, Blackwell HE, and Grubbs RH J.Am. Chem. Soc 118 1996
Synthesis of Cyclic Peptides by Ring-Closing Metathesis Reichwein JF, Versluis C, and Liskamp RMJ J.Org. Chem 65 2000
Non-Standard Amino Acids in Peptide Design and Protein Engineering Balaram P Curr. Opin. Struct. Biol 2 1992
Control of Peptide Conformation by the Thorpe-Ingold Effect (C-alpha Tetrasubstitution) Toniolo C, Crisma M, Formaggio F and Peggion C Biopolymers 60 2001
Protease Inhibitors: Current Status and Future Prospects Leung D, Abbenante G and Fairlie DP J. Med. Chem 43 2000
book
Spatola AF Chemistry and Biochemistry of Amino Acids, Peptides and Proteins, Vol. III Marcel Dekker 1983
Phenol¶
Below are shown some possible isosteric replacements for the phenol moiety.
articles
Estrogen receptor beta-subtype selective tetrahydrofluorenones: Use of a fused pyrazole as a phenol bioisostere R.R. Wilkening, R.W. Ratcliffe, A.K. Fried, D. Meng, W. Sun, L. Colwell, S. Lambert, M. Greenlee, S. Nilsson, A. Thorsell et al. Bioorganic and Medicinal Chemistry Letters 16(15) 2006
Pyridine¶
There are several possible bioisosteric replacements for the pyridine moiety, as shown in the figure below.
articles
Isosterism and molecular modification in drug design Thornber C.W. Chem.Soc.Rev 8 1979
Cyclopropylamino acid amide as a pharmacophoric replacement for 2,3-diaminopyridine. Application to the design of novel bradykinin B1 receptor antagonists Wood MR, Schirripa KM, Kim JJ, Wan BL, Murphy KL, Ransom RW, Chang RS, Tang C, Prueksaritanont T, Detwiler TJ, Hettrick LA, Landis ER, Leonard YM, Krueger JA, Lewis SD, Pettibone DJ, Freidinger RM, Bock MG. J Med Chem 49(4) 2006
book
Richard Silverman The organic chemistry of drug design and drug action Elsevier, Academic Press 2004
Ring¶
Many bioisosteric replacements are possible for a ring. Aromatic, heterocyclic and aliphatic ring systems can be replaced by similar equivalent moieties.
book
Richard Silverman The organic chemistry of drug design and drug action Elsevier, Academic Press 2004
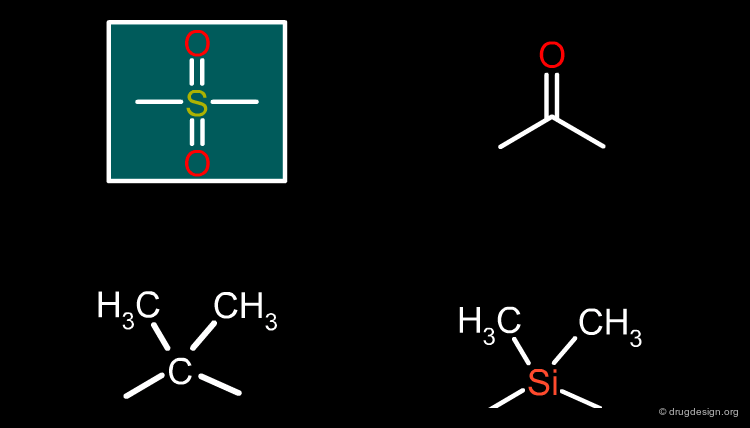
Sulfonyl¶
A carbonyl is a possible bioisostere for the replacement of a sulfonyl group. Other replacements include the gem-dimethyl group or its silicon equivalent.
articles
Chemistry challenges in lead optimization: silicon isosteres in drug discovery Graham A. Showell and John S. Mills Drug Discovery Today 8 (12) 2003
Spacer¶
Examples of aliphatic spacer moieties replaced by bioisosteric ring structures are indicated below.
articles
Isosterism and molecular modification in drug design Thornber C.W. Chem.Soc.Rev 8 1979
book
Richard Silverman The organic chemistry of drug design and drug action Elsevier, Academic Press 2004
Thioether¶
Several possibilities exist for the bioisoteric replacement of a thioether group, as indicated in the figure below.
articles
Isosterism and molecular modification in drug design Thornber C.W. Chem.Soc.Rev 8 1979
book
Richard Silverman The organic chemistry of drug design and drug action Elsevier, Academic Press 2004
Thiourea¶
In the figure below are shown various possible bioisosteric replacements for the thiourea group.
articles
Isosterism and molecular modification in drug design Thornber C.W. Chem.Soc.Rev 8 1979
book
Richard Silverman The organic chemistry of drug design and drug action Elsevier, Academic Press 2004
Examples of Bioisosteric Transformations¶
Four Types of Bioisosteric Transformations¶
There are four types of bioisosteric transformations, which will be presented with examples in the following pages. In most cases they correspond to successful attempts; however, we do not discuss the biological activities obtained. The examples are shown simply to illustrate some bioisosteric replacements used in various projects.
Ring-to-Ring Transformations¶
Three examples of ring-to-ring bioisosteric transformations are shown in the following pages.
Example 1¶
In the example shown here the quinazoline heterocycle (reference) was first replaced by a quinoline ring (design-1) and then by an isoquinoline bioisostere heterocycle (design-2).
articles
The 4-anilinoquinazoline class of inhibitors of the erbB family of receptor tyrosine kinases Denny WA Il Farmaco 56 2001
Tyrosine kinase inhibitors. 16. 6,5,6-tricyclic benzothieno[3, 2-d]pyrimidines and pyrimido[5,4-b-] and -[4,5-b]indoles as potent inhibitors of the epidermal growth factor receptor tyrosine kinase Showalter HD, Bridges AJ, Zhou H, Sercel AD, McMichael A, Fry DW J. Med. Chem 42 1999
Chemical Inhibitors of Protein Kinases Bridges AJ Chem. Rev 101 2001
Protein kinase inhibitors: structural determinants for target specificity McMahon G, Sun L, Liang C and Tang C Current Opinion in Drug Discovery and Development 1 (2) 1998
Example 2¶
The quinolin-4(1H) bicycle of the starting compound (reference) was transformed into a naphtyridin-4(1H) heterocycle (design-1), a pyrimido[1,2a] pyrimidin-4 bicycle (design-2) and a pyrido[1,2a] pyrimidin-4(1H) bioisostere moiety (design-3).
articles
In vitro preclinical lead optimisation technologies (PLOTs) in pharmaceutical development Atterwill CK, Wing MG. Toxicol Lett 127 (1-3) 2002
Example 3¶
In this example the initial 4H-thieno [3,2b] pyrrole (reference) was modified into a 6H-thieno [2,3b] pyrrole bicycle (design-1) and also into a 1H-indole bioisostere system (design-2).
articles
Thieno[3,2-b]- and Thieno[2,3-b]pyrrole Bioisosteric Analogues of the Hallucinogen and Serotonin Agonist N,N-Dimethyltryptamine Joseph B. Blair, Danuta Marona-Lewicka, Arthi Kanthasamy, Virginia L. Lucaites, David L. Nelson, and David E. Nichols J. Med. Chem 42 1999
Chain-to-Ring Transformations¶
Three examples of chain-to-ring bioisosteric transformations are shown in the following pages.
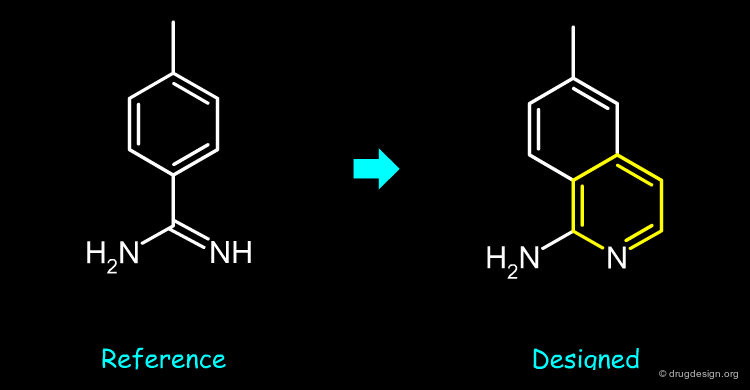
Example 1¶
The initial carboximidamide (reference) was modified into an isoquinoline-1-amine bioisostere (designed).
articles
Design and SAR of novel potassium channel openers targeted for urge urinary incontinence. 1. N-Cyanoguanidine bioisosteres possessing in vivo bladder selectivity Butera JA, Antane MM, Antane SA, Argentieri TM, Freeden C, Graceffa RF, Hirth BH, Jenkins D, Lennox JR, Matelan E, Norton NW, Quagliato D, Sheldon JH, Spinelli W, Warga D, Wojdan A, Woods M. J Med Chem 43 (6) 2000
Example 2¶
The ester group of the initial structure (reference) was replaced by an oxadiazole bioisostere (designed).
Example 3¶
In this example, the design considered the initial diethylamine molecule (reference) to be cyclicized into a piperidine bioisostere analog (designed).
articles
Structure-based optimization of MurF inhibitors Stamper GF, Longenecker KL, Fry EH, Jakob CG, Florjancic AS, Gu YG, Anderson DD, Cooper CS, Zhang T, Clark RF, Cia Y, Black-Schaefer CL, Owen McCall J, Lerner CG, Hajduk PJ, Beutel BA, Stoll VS Chem Biol Drug Des 67 (1) 2006
Predicting mosquito repellent potency of N,N-diethyl-m-toluamide (DEET) analogs from molecular electronic properties Ma D, Bhattacharjee AK, Gupta RK, Karle JM Am J Trop Med Hyg 60(1) 1999
Minimum structural requirements for minoxidil inhibition of lysyl hydroxylase in cultured fibroblasts Murad S, Walker LC, Tajima S, Pinnell SR Arch Biochem Biophys 308(1) 1994
Pharmacological characterization of novel water-soluble cannabinoids Martin BR, Wiley JL, Beletskaya I, Sim-Selley LJ, Smith FL, Dewey WL, Cottney J, Adams J, Baker J, Hill D, Saha B, Zerkowski J, Mahadevan A, Razdan RK J Pharmacol Exp Ther 318(3) 2006
Ring-to-Chain Transformations¶
Three examples of ring-to-chain bioisosteric transformations are shown in the following pages.
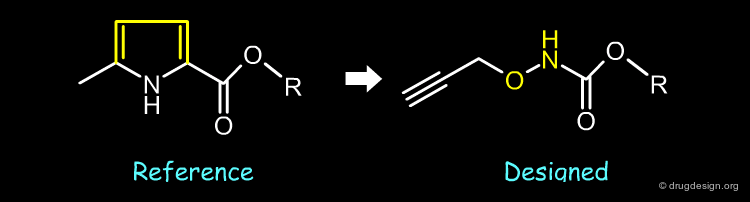
Example 1¶
The pyrrole moiety of the reference compound was replaced by a propynylhydroxyamino bioisostere.
articles
Coumarin inhibitors of gyrase B with N-propargyloxy-carbamate as an effective pyrrole bioisostere Periers AM, Laurin P, Ferroud D, Haesslein JL, Klich M, Dupuis-Hamelin C, Mauvais P, Lassaigne P, Bonnefoy A, Musicki B. Bioorg Med Chem Lett 10 (2) 2000
Example 2¶
The reference amino-quinazoline molecule with a bicyclic moiety was replaced by a salicylamide bioisostere.
articles
Salicylanilides as inhibitors of the protein tyrosine kinase epidermal growth factor receptor Liechti C, Sequin U, Bold G, Furet P, Meyer T, Traxler P Eur. J. Med. Chem 39 2004
Tyrosine kinase inhibitors. 8. An unusually steep structure-activity relationship for analogues of 4-(3-bromoanilino)-6,7-dimethoxyquinazoline (PD 153035), a potent inhibitor of the epidermal growth factor receptor Bridges AJ, Zhou H, Cody DR, Rewcastle GW, McMichael A, Showalter HD, Fry DW, Kraker AJ, Denny WA j. Med. Chem 39 1996
A diazine heterocycle replaces a six-membered hydrogen-bonded array in the active site of scytalone dehydratase Hodge CN and Pierce J Bioorganic and Medicinal Chemistry Letters 3 1993
From arylureas to biarylamides to aminoquinazolines: Discovery of a novel, potent TRPV1 antagonist Xiaozhang Zheng, Kevin J. Hodgetts, Harry Brielmann, Alan Hutchison, Frank Burkamp, A. Brian Jones, Peter Blurton, Robert Clarkson, Jayaraman Chandrasekhar, Rajagopal Bakthavatchalam, Stephane De Lombaert, Marci Crandall, Daniel Cortrighta and Charles A. Bluma Bioorganic and Medicinal Chemistry Letters 16 2006
Example 3¶
In this example the initial phenyltriazolo-benzodiazepine molecule having a tricyclic moiety was replaced by a phenoxyphenyl-triazole bioisostere.
articles
Design and Synthesis of 4H-3-(2-Phenoxy)phenyl-1,2,4-triazole Derivatives as Benzodiazepine Receptor Agonists Akbarzadeh T, Tabatabai SA, Khoshnoud MJ, Shafaghic B and Shafiee A Bioorganic and Medicinal Chemistry 11 2003
Chain-to-Chain Transformations¶
Three examples of chain-to-chain bioisosteric transformations are shown in the following pages.
Example 1¶
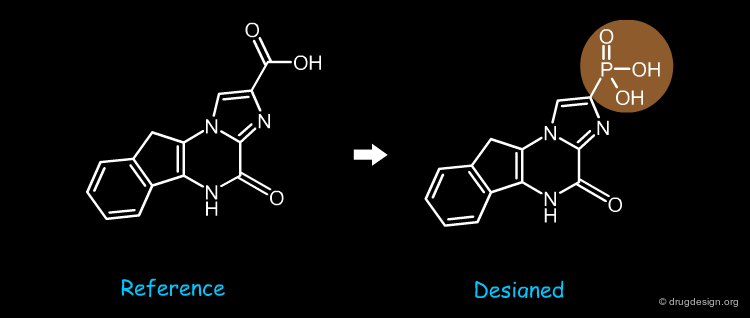
The carboxylic acid of the reference molecule was replaced by a phosphonic acid bioisostere.
articles
Bioisosteres of 9-carboxymethyl-4-oxo-imidazo[1,2-a]indeno-[1,2-e]pyrazin-2-carboxylic acid derivatives. Progress towards selective, potent in vivo AMPA antagonists with longer durations of action Jimonet P, et al. Bioorg Med Chem Lett 11(2) 2001 10.1016/S0960-894X(00)00592-8
Example 2¶
Here, the N-propylamine moiety of the reference compound was replaced by an equivalent hydrazine bioisostere.
articles
Amidinohydrazones as guanidine bioisosteres: application to a new class of potent, selective and orally bioavailable, non-amide-based small-molecule thrombin inhibitors Soll RM, Lu T, Tomczuk B, Illig CR, Fedde C, Eisennagel S, Bone R, Murphy L, Spurlino J, Salemme FR. Bioorg Med Chem Lett 10 (1) 2000
Example 3¶
In this example the amide moiety of the reference compound was replaced by an equivalent urea bioisostere.
articles
Structure-Based Generation of a New Class of Potent Cdk4 Inhibitors: New de Novo Design Strategy and Library Design Honma T, Hayashi K, Aoyama T, Hashimoto N, Machida T, Fukasawa K, Iwama T, Ikeura C, Ikuta M, Suzuki-Takahashi I, Iwasawa Y, Hayama T, Nishimura S and Morishima H J. Med. Chem 44 2001
A Novel Approach for the Development of Selective Cdk4 Inhibitors: Library Design Based on Locations of Cdk4 Specific Amino Acid Residues Honma T, Yoshizumi T, Hashimoto N, Hayashi K, Kawanishi N, Fukasawa K, Takaki T,Ikeura C, Ikuta M, Suzuki-Takahashi I, Hayama T, Nishimura S and Morishima H J. Med. Chem 44 2001
Commercial Bioisosteric Drugs: Examples¶
Angiotensin Receptor Blockers (ARBs)¶
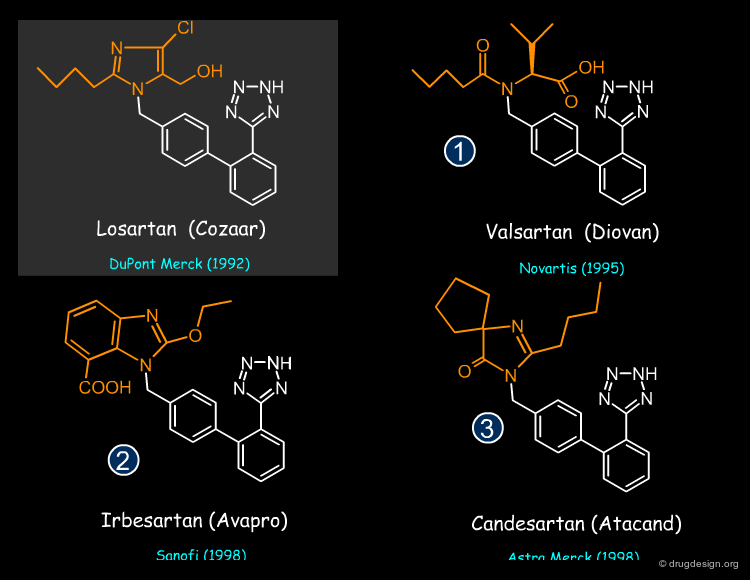
Valsartan, Irbesartan and Candesartan are all bioisoteres of the Losartan Angiotensin Receptor Blocker and replaced the imidazole moiety of this drug by various bioisosteric groups (patent priorities are indicated in parenthesis).
book
The Merck Index Published by: Merck and Co. Whitehouse Station, NJ 1996
COX-2 Inhibitors¶
Vioxx, and Bextra are examples of COX-2 inhibitor drugs whose structures were derived from that of Celebrex using the concept of bioisosterism (patent priorities are indicated in parenthesis).
book
The Merck Index Published by: Merck and Co. Whitehouse Station, NJ 1996
Anti-Inflammatory NSAIDs¶
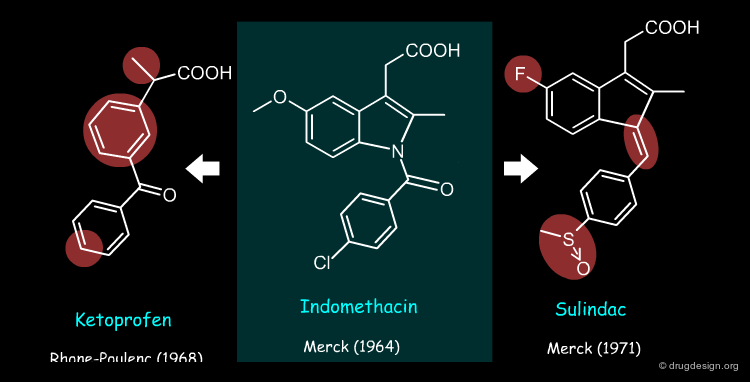
Sulindac and Ketoprofen are examples of non-steroid anti-inflammatory drugs (NSAIDs) whose structure was derived from that of Indomethacin by using the concept of bioisosterism (patent priorities are indicated in parenthesis).
book
The Merck Index Published by: Merck and Co. Whitehouse Station, NJ 1996
Antiarrhytmic Beta-Adrenergics¶
Pindolol and Atenolol are examples of antiarrhytmic beta-adrenergic drugs whose structure was derived from that of Propranolol by using the concept of bioisosterism (patent priorities are indicated in parenthesis).
book
The Merck Index Published by: Merck and Co. Whitehouse Station, NJ 1996
Neuroleptics¶
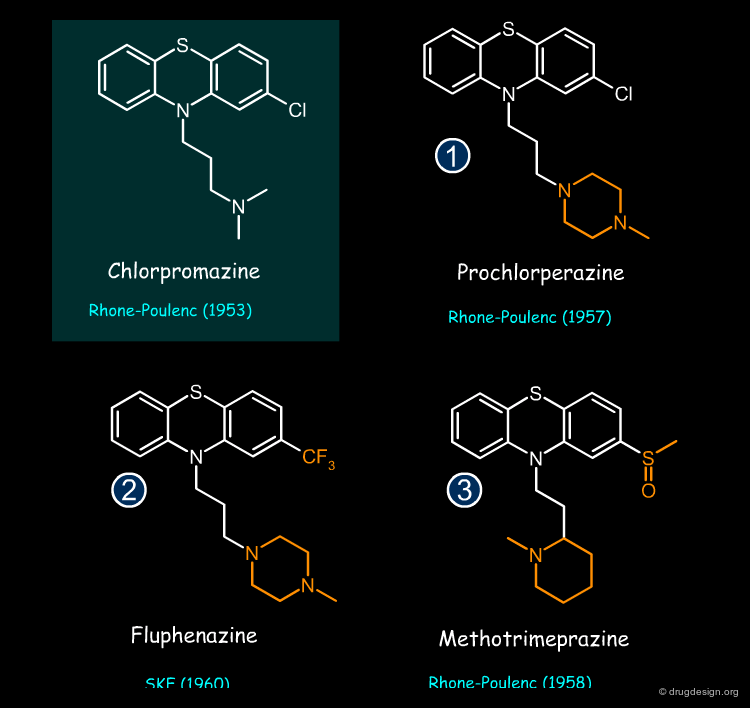
Prochloperazine, Fluphenazine and Methotrimeprazine are examples of neuroleptic drugs whose structure was derived from that of Chlorpromazine by using the concept of bioisosterism (patent priorities are indicated in parenthesis).
book
The Merck Index Published by: Merck and Co. Whitehouse Station, NJ 1996
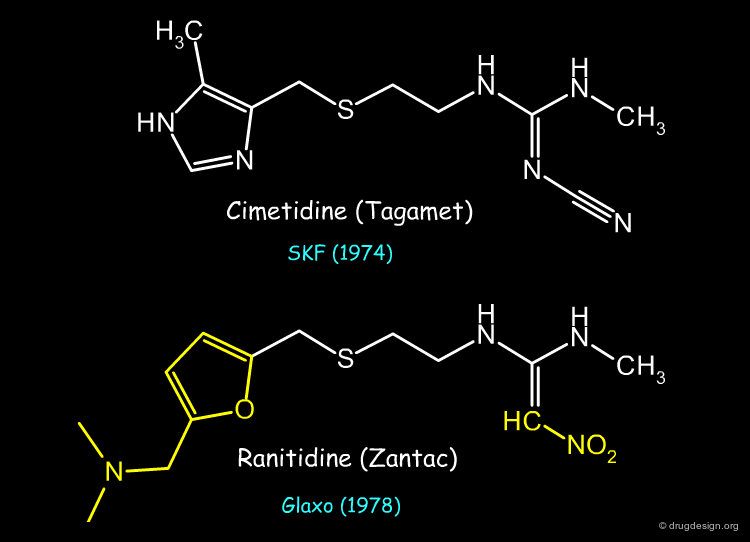
Anti-Ulcers¶
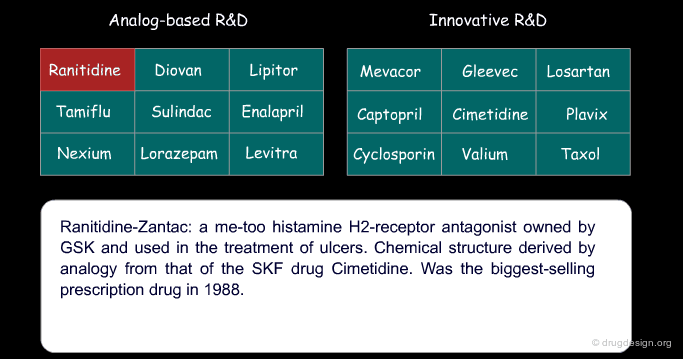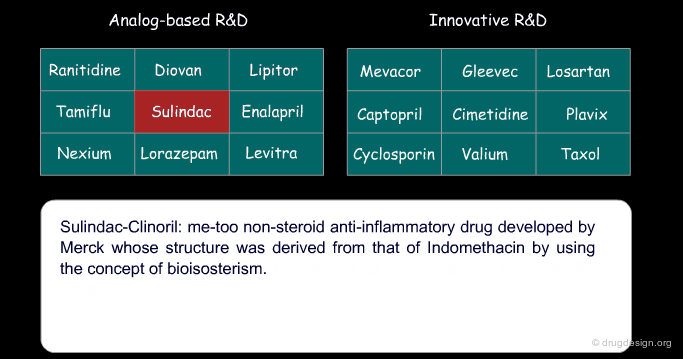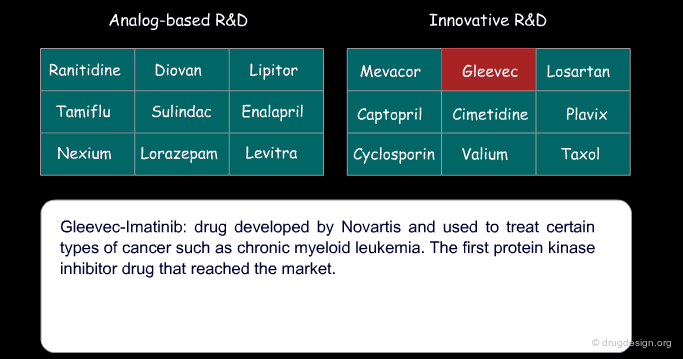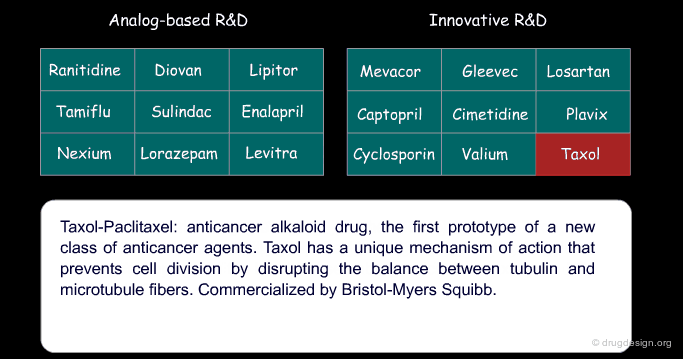
Ranitidine (Zantac) is an example of an anti-ulcer drug whose structure was derived from that of Cimetidine (Tagamet) by using the concept of bioisosterism (patent priorities are indicated in parenthesis).
book
The Merck Index Published by: Merck and Co. Whitehouse Station, NJ 1996
Male Erectile Dysfunction Drugs¶
Levitra (Vardenafil) is an example of a male erectile dysfunction drug whose structure was derived from that of Viagra (Sildenafil) by using the concept of bioisosterism (the FDA approval dates are indicated in parenthesis).
book
The Merck Index Published by: Merck and Co. Whitehouse Station, NJ 1996
Benzodiazepines¶
Nitrazepam, Prazepam and Clobazam are examples of anxiolytic drugs whose structures were derived from that of Diazepam (Valium) by using the concept of bioisosterism (patent priorities are indicated in parenthesis).
book
The Merck Index Published by: Merck and Co. Whitehouse Station, NJ 1996
Antibacterial Sulfonamides¶
Sulfacytine, Sulfaperine, Sulfamethazine and Sulfathiazole are examples of antibacterial drugs whose structures were derived from that of a reference sulfonamide compound (Sulfabenz - 1908) by using, at a given stage, the concept of bioisosterism (patent priorities are indicated in parenthesis).
book
The Merck Index Published by: Merck and Co. Whitehouse Station, NJ 1996
Beta-Lactam Antibiotics¶
Penicillin G, Amoxicillin, Ampicillin and Penicillin V are examples of beta-lactam antibiotics that are all bioisosteres (patent priorities are indicated in parenthesis).
book
The Merck Index Published by: Merck and Co. Whitehouse Station, NJ 1996
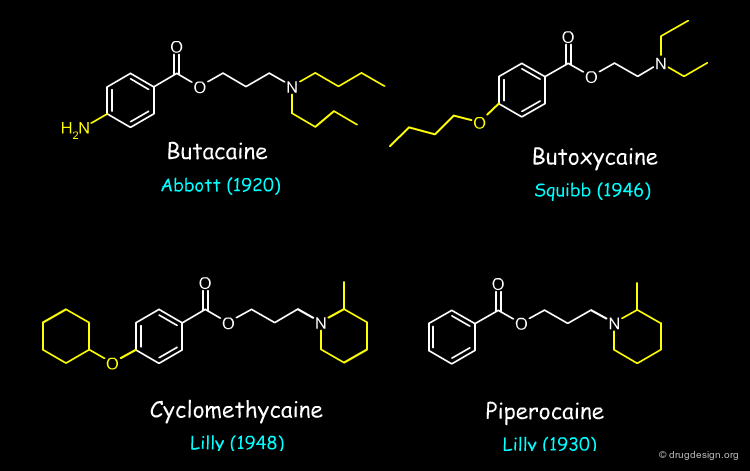
Local Anesthetics¶
Butacaine, Butoxycaine, Cyclomethycaine and Piperocaine are examples of local anesthetic drugs whose structures were derived from a reference lead compound by using, at a given stage, the concept of bioisosterism (patent priorities are indicated in parenthesis).
book
The Merck Index Published by: Merck and Co. Whitehouse Station, NJ 1996
Glucocorticoid Steroids¶
Prednisone, Corticosterone, Dexamethazone and Cortisone are examples of glucocorticoid drugs whose structures were derived from a steroid lead compound by using, at a given stage, the concept of bioisosterism (patent priorities are indicated in parenthesis).
book
The Merck Index Published by: Merck and Co. Whitehouse Station, NJ 1996
Statin Drugs¶
Statins are hypolipidemic agents, used to lower cholesterol levels (by inhibition of the HMG-CoA reductase) to reduce the risk of cardiovascular disease. There are two groups of statins: fermentation derived drugs and synthetic drugs. Examples of bioisostere statin drugs are indicated below for each group (commercialization dates are in parenthesis).
articles
Lovastatin and beyond: the history of the HMG-CoA reductase inhibitors Tobert JA Nat Rev Drug Discov 2(7) 2003
Rosuvastatin calcium Quirk J, Thornton M, Kirkpatrick P. Nat Rev Drug Discov 2(10) 2003
Patent Issues with Bioisosterism¶
Limits of Patent Infringements on Structures?¶
What are the limits of patent infringements on chemical structures? Look for example at the benzodiazepine anxiolytic drugs developed by the pharmaceutical companies indicated below. The chemical formulas are indeed very similar and close to that of Valium; however Warner-Lambert, Sumitomo, Schering and Delmar had no problem developing or marketing their own analogs.
book
The Merck Index Published by: Merck and Co. Whitehouse Station, NJ 1996
The Viagra-Levitra Case¶
Bioisosterism makes it very difficult for a pharmaceutical company to sue a competitor for copying the chemical structure of its proprietary drug. For example Pfizer sued Bayer and GlaxoSmithKline for infringement on the Viagra patent; however the case failed both in Europe and in the USA (the FDA approval dates are indicated in parentheses).
articles
Vardenafil Bayer Yakuhin Sommer F, Engelmann U. Curr Opin Investig Drugs 3(4) 2002
Comparison of phosphodiesterase type 5 (PDE5) inhibitors Wright PJ. Int J Clin Pract
. 2006
The Diazepam-Clobazam Example¶
Clobazam is a drug whose structure was intelligently derived from that of Diazepam (Valium) by replacing the azomethine group of diazepam by an equivalent planar amide. This bioisosteric transformation led to a drug with an original chemical structure that was fully patentable, with no possible dispute with Roche (note that one tautomer of clobazam could have been invoked as infringing the Diazepam patent).
articles
Study of an anxiolytic, clobazam, in otorhinolaryngology in psychosomatic pharyngeal manifestations (in French) Freche, C. Semaine des Hopitaux; Therapeutique 51 (4) 1975
Patent Issues with Chiral Enantiomers¶
It is often assumed that a chiral-switch cannot lead to patentable drugs, but this is not always true. Fluoxetine (Prozac) is an example where the owner of the racemate (Eli-Lilly) signed a license agreement with his rival (Sepracor) to have the right to develop one enantiomer of its own drug! There are no general rules in this field and disputes have to be treated case by case.
articles
Intellectual property and chirality of drugs Israel Agranat and Hava Caner Drug Discovery Today 4 (7) 1999
Putting chirality to work: the strategy of chiral switches Agranat I, Caner H, Caldwell J. Nat Rev Drug Discov 1 (10) 2002
Sepracor/Eli-Lilly License Agreement Sepracor Eli-Lilly SCRIP 8 1998
Patentable Drugs by Bioisosterism¶
Patent issues are not always straightforward; however, when conducted in an intelligent manner bioisosterism provides a method of choice for unequivocally designing patentable drugs whose structures are derived from the structures of the competitor's drugs.
Programs and Databases on Bioisosterism¶
Computerized Systems¶
Many computer programs have been developed for similarity searching purposes (see chapters on similarity searching and those on database searching); however only a few have been built strictly using the concept of bioisosterism. Emil and Bioisoster/Bioster were the first programs and Brood is one of the most recent software products developed along this line; they can perform automated bioisosteric transformations of entire molecules.
EMIL Program¶
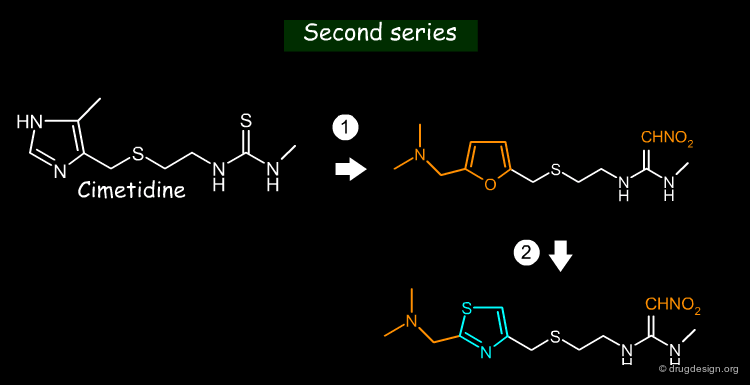
EMIL (Example Mediated Innovation for Lead Evolution) is an expert system developed in Japan by Toshio Fujita (1993). It is based on a "lead evolution" principle which consists of making consecutive structural modifications of an initial molecule to obtain new generation compounds. The program incorporates a database of known examples together with more than 2000 rules for bioisosteric molecular transformations. Below are shown structures generated with Cimetidine as a starting point.
articles
Similarities in bioanalogous structural transformation patterns among various bioactive compound series Fujita T Biosci. Biotech. Biochem 60(4) 1996
book
Toshio Fujita Trends in QSAR and Molecular Modeling 92. Procedings of the 9th European Symposium on Structure-Activity Relationships: QSAR and Molecular Modelling ESCOM - Leiden 1993
Toshio Fujita et al. Kyoto University and EMIL PROJECT, Fujitsu Kansai Systems Laboratory, Osaka, Japan QSAR and Drug Design: New developments and applications Elsevier, Pharmacochemistry Library Volume 23, H. Timmerman Ed. 1995
Toshio Fujita ACS Symposium series No. 606 Classical and Three-Dimensional QSAR in Agrochemistry The American Chemical Society 1995
BIOISOSTER Program and BIOSTER Database¶
The BIOISOSTER program (Balakin et al, 2004) and its BIOSTER database (Ujivary, 1997) is an example of computerized tools that were developed independently of the EMIL software, with however many similarities between the two systems.
articles
Bioisosteric morphing in primary hit optimization KV Balakin, SE Tkachenko, I Okun, AV Skorenko, YA Ivanenkov, NP Savchuk, AA Ivashchenko and Y Nikolsky Chimica Oggi - Chemistry Today Jan-Feb 2004
Extended Summary: BIOSTER - a database of structurally analogous compounds Ujvary I Pesticide Science 51 (1) 1997
The BIOISOSTER Program¶
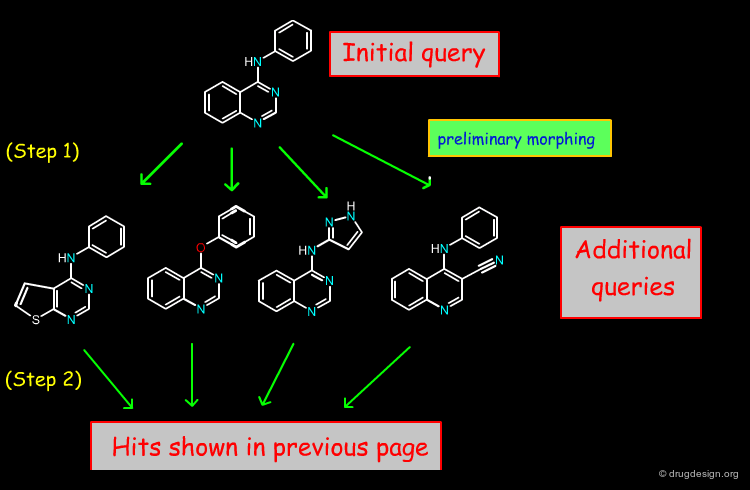
The BIOISOSTER program developed by Balakin et al (2004) can be used to find bioisosteric analogs in 2D structural databases. The program can also generate chemical libraries conforming to bioisosteric transformation rules (that are target specific and customizable). In the example below are illustrated the types of hits that were obtained starting with a 4-anilinoquinazoline query.
articles
Bioisosteric morphing in primary hit optimization KV Balakin, SE Tkachenko, I Okun, AV Skorenko, YA Ivanenkov, NP Savchuk, AA Ivashchenko and Y Nikolsky Chimica Oggi - Chemistry Today Jan-Feb 2004
Bioisosteric Morphing of the Query¶
The BIOISOSTER algorithm for finding bioisosteric analogs in a structural database is a two-step procedure. (1) In the first step the query input is "morphed" using a set of bioisosteric rules, and (2) in the second step the transformed structures are used as queries for searching in the database. Below step (1) is illustrated (morphing treatment), that was developed for the anilinoquinazoline query of the previous page.
articles
Bioisosteric morphing in primary hit optimization KV Balakin, SE Tkachenko, I Okun, AV Skorenko, YA Ivanenkov, NP Savchuk, AA Ivashchenko and Y Nikolsky Chimica Oggi - Chemistry Today Jan-Feb 2004
The Accelrys BIOSTER Database¶
The BIOSTER database consists of a systematic compilation of successful bioisosteric replacements that were described in medicinal chemistry articles in the last 35 years (up to 2002). This database contains about 14,000 pairs of bioisosteres together with the bioisosteric relationships between pairs of compounds.
articles
Extended Summary: BIOSTER - a database of structurally analogous compounds Ujvary I Pesticide Science 51 (1) 1997
Example of BIOSTER Database Content¶
Below is illustrated an output of the Accelrys Bioster database showing the bioisosteric transformation of an initial molecule having a carboxylic acid group into a sulfonamide moiety. The program gives the calculated LogP for the two compounds as well as those of the elementary fragments. References from the literature associated with this type of transformation are also given.
BROOD Program¶
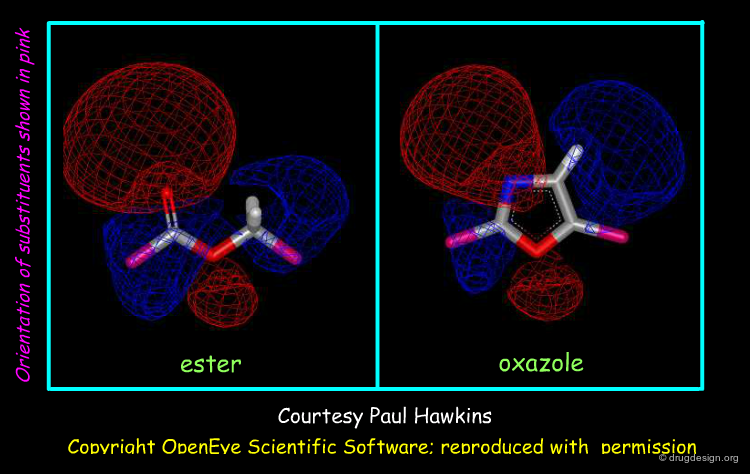
Brood is a recent program developed by OpenEye for bioisostere prediction. It identifies bioisosteres by finding fragments with shape, chemistry and electrostatics that are similar to a 3D query molecule. Below is visualized the identification of an oxazole having similar electrostatic isopotential contour surfaces as compared to an ester moiety (red regions are negative and blue regions are positive). For electrostatics the Tanimoto coefficient is 0.54 whereas it is 0.90 for shape similarity.
Brood Program for Bradykinin Antagonists¶
Paul Hawkins (OpenEye Scientific Software Inc.) used the Brood program to find bioisoteres of a toxic bradykinin antagonist. He used the toxic 2-3-diaminopyridine moiety as a query. Indeed, the cyclopropyl carbonyl hit corresponds to a potent antagonist recently discovered at Merck. This confirms Brood's ability to generate useful bioisosteres and encourages studying the other structures generated.
articles
Cyclopropylamino acid amide as a pharmacophoric replacement for 2,3-diaminopyridine. Application to the design of novel bradykinin B1 receptor antagonists Wood MR, Schirripa KM, Kim JJ, Wan BL, Murphy KL, Ransom RW, Chang RS, Tang C, Prueksaritanont T, Detwiler TJ, Hettrick LA, Landis ER, Leonard YM, Krueger JA, Lewis SD, Pettibone DJ, Freidinger RM, Bock MG. J Med Chem 49(4) 2006
OpenEye Scientific Software Inc P. Hawkins
Personnal communication
Organon IBIS Program¶
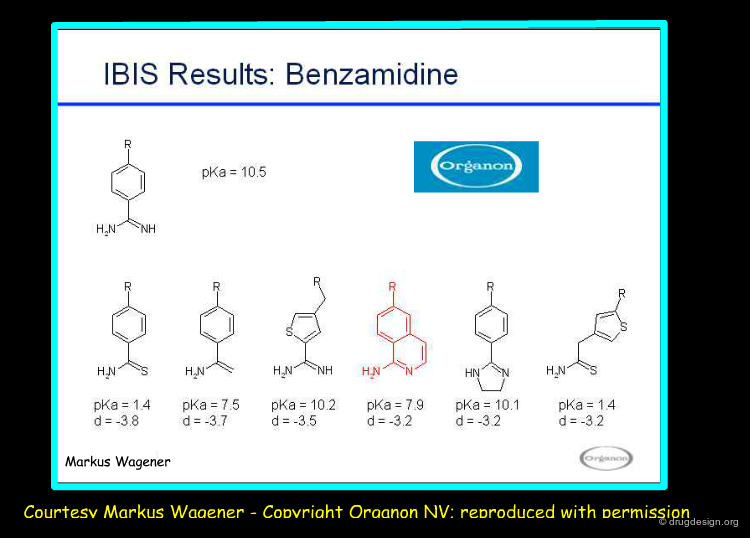
Organon has developed an in-house intranet application program called IBIS to search for bioisosteric replacements. Constraints such as LogP and pKa allow for focusing the search. The performance of the method has been validated using bioisosteric fragment pairs extracted from the BIOSTER database. In the example below are shown bioisosteres of benzamidine.
articles
The quest for bioisosteric replacements M Wagener and JPM Lommerse J Chem Inf Model 46(2) 2006
Novartis Program for Ring Bioisosteres¶
Novartis developed an in-house program based on neural networks enabling the identification of novel bioisosteric ring analogs. The parameters that are considered for bioisosterism can include energy, molecular weight and atomic charges. In the example illustrated below, bioisosteres identified for a benzothiazole target ring are displayed.
articles
Quest for the rings. In silico exploration of ring universe to identify novel bioactive heteroaromatic scaffolds Ertl P, Jelfs S, Muhlbacher J, Schuffenhauer A, Selzer P. J Med Chem 49(15) 2006
Web-based cheminformatics and molecular property prediction tools supporting drug design and development at Novartis P. Ertl, J. Muehlbacher. B. Rohde, P. Selzer SAR and QSAR Env. Res 14 2003
GlaxoSmithKline Program for Ring Replacements¶
GlaxoSmithKline developed a ring database to be used for bioisosteric replacements. The program displays the frequency of occurrence in the corporate database (as an indicator of synthetic accessibility) together with similarity values. Below is illustrated the output obtained with the indole query.
articles
Drug rings database with web interface. A tool for identifying alternative chemical rings in lead discovery programs Lewell XQ, Jones AC, Bruce CL, Harper G, Jones MM, McLay IM, Bradshaw J. J Med Chem 46(15) 2003
COSMOsim Program for Bioisosteric Similarity¶
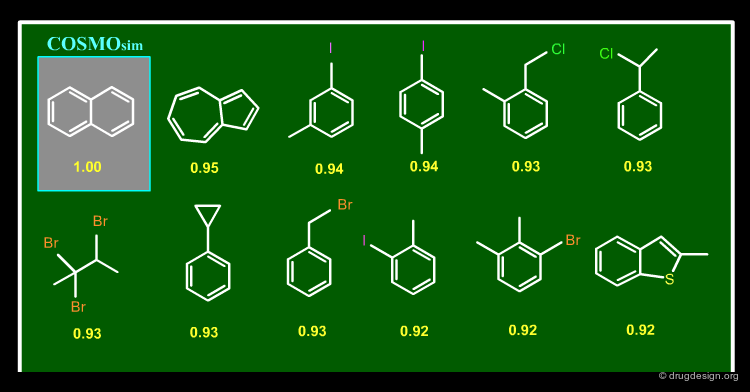
A program called COSMOsim has been developed based on the calculation of polarization charge densities where the molecules are considered as swimming in a perfect conductor. Quantum mechanical calculations are used for generating Σ profiles (histograms) that enable the calculation of "Σ-match similarities". Examples of bioisosteres obtained for the naphthalene query are shown in the figure below (similarity scores are indicated in yellow).
articles
COSMOsim: bioisosteric similarity based on COSMO-RS sigma profiles Thormann M, Klamt A, Hornig M, Almstetter M. J Chem Inf Model 46(3) 2006
Cheminformatics Software¶
Cheminformatics led to the development of tools based on 2D descriptors, connectivity tables, Tanimoto coefficients, structural 2D/3D fingerprints etc., to treat molecular similarity in a quantitative manner. This is beyond the classical concept of bioisosterism and will be discussed in the chapter on "Molecular Similarity". Commercial software includes Daylight, MDL, Tripos, Accelrys and CAS programs.
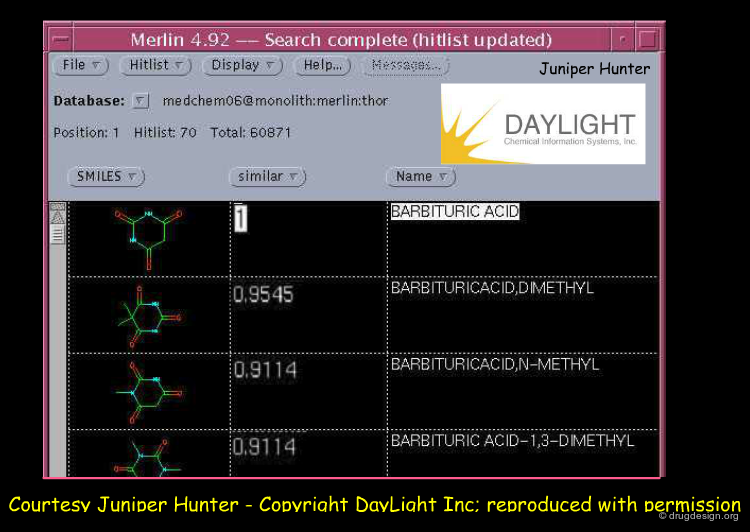
Daylight: MERLIN Program¶
MERLIN is an example of a bioinformatics program developed by Daylight Chemical Information Systems Inc. for searching, sorting and selecting molecules from a database. Below is shown the output obtained by searching similar compounds using barbituric acid as a query (the molecules are sorted in terms of the Tanimoto similarity coefficient).
Tripos: SYBYL Platform¶
Tripos has several cheminformatics tools that make use of 2D similarity and include different measurements of similarity such as Tanimoto, Asymmetric or Cosine similarity coefficients. The following views illustrate screen snapshots obtained using Unity (with Tanimoto coefficients) and Benchware Dataminer.
MDL: ISENTRIS Program¶
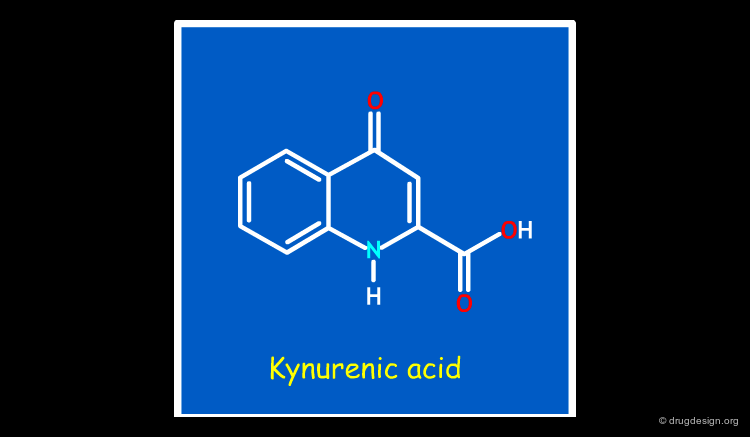
MDL has developed a cheminformatics platform named Isentris for managing scientific information. In the example below, Kynurenic acid was used as a query to search in the MDL Drug Data Report (MDDR) database containing over 132,000 substances. Typical hits are shown below with their similarity index (MolSim) and their main biological property (Activity).
MDL: DiscoveryGate Program¶
In the example below the MDL program DiscoveryGate was used to search for isosteres of naphthalene. The search yielded 670 compounds; the first nine structures are shown below.
CAS: SciFinder Program¶
SciFinder is a research platform developed by CAS that can be used to access information in journals and patent literature. The example below illustrates the structure of a drug which was used to probe for bioisosteric candidates in the literature (Set-1). In the second step the query structure was used to search only for compounds with biological studies available in the literature (Set-2).
Review Articles and Books¶
Review Articles on Bioisosterism¶
Despite the importance of bioisosterism, there are relatively few systematic review articles on this field. Below are indicated some articles that have been written on bioisosterism, ranked in reverse chronological order. The full references appear in the "additional information" area at the bottom left side of this page.
articles
Bioisosterism: a useful strategy for molecular modification and drug design Lima LM, Barreiro EJ. Curr Med Chem 12(1) 2005
Chemical similarity and biological activities Kubinyi H J Braz Chem Soc 13(6) 2002
The use of bioisosteric groups in lead optimization Olesen PH Curr Opin Drug Discov Devel 4(4) 2001
Bioisosterism and Molecular Diversity Robert D. Clark, Allan M. Ferguson, and Richard D. Cramer Perspectives in Drug Discovery and Design 9-10-11 1998
Bioisosterism as a molecular diversity descriptor: steric fields of single "topomeric" conformers Cramer RD, Clark RD, Patterson DE, Ferguson AM. J Med Chem 39(16) 1996
Bioisosterism: A Rational Approach in Drug Design Patani GA, LaVoie EJ. Chem Rev 96(8) 1996
Bioisosterism in drug design Lipinski CA Ann. Rep. Med. Chem 21 1986
Isosterism and molecular modification in drug design Thornber C.W. Chem.Soc.Rev 8 1979
Bioisosterism Hansch C , Intra-Science Chem. Report 8 1974
book
Burger A. Prog. Drug. Res. Vol. 37 Birkhauser Publ. 1991
Books on Bioisosterism¶
Below are indicated books that have been written (entirely or in part) on bioisosterism, ranked in reverse chronological order. The full references appear in the "additional information" area at the bottom left side of this page.
book
Janos Fischer and Robin C. Ganellin Analogue-based Drug Discovery Wiley-VCH 2006
Richard Silverman The organic chemistry of drug design and drug action Elsevier, Academic Press 2004
C. G. Wermuth
Academic Press 1996
Ariens EJ Vol. 1 Academic Press, New York 1971
Korolkovas A. Essentials of Molecular Pharmacology: Background for Drug Design Wiley 1970
Foye WO
1970
Schatz VB Medicinal Chemistry Wiley-Interscience 1960
Limitations and the Future¶
The Receptor is the Ultimate Decider¶
In bioisosterism a successful replacement is never guaranteed because the receptor is the ultimate "decider" and this is illustrated here. In the area of β-adrenergic agents it has been proven that the replacement of a phenolic hydroxyl with a sulfonamide group retains the biological activities, and this was attributed to the similar pKa values of the two groups. However for opioid ligands, the same replacement led to inactive molecules.
articles
Investigation of Phenolic Bioisosterism in Opiates: 3-Sulfonamido Analogues of Naltrexone and Oxymorphone Christopher R. McCurdy, Robert M. Jones, Philip S. Portoghese Organic Letters 2(6) 2000
A new bio-isostere: alkylsulfonamidophenethanolamines A.A. Larsen, P.M. Lish Nature 203 1964
The Multidimensional Nature of Bioisosterism¶
As Thornber points out, there are many parameters that have to be considered when deciding on a bioisosteric replacement and any replacement is unlikely to leave all these parameters unmodified. These parameters include size, electronic distribution, lipid solubility, water solubility, pKa, chemical reactivity (e.g. likelihood of metabolism) and hydrogen bond capacity. In the following pages we visualize some of these properties for the three molecules shown below.
articles
Isosterism and molecular modification in drug design Thornber C.W. Chem.Soc.Rev 8 1979
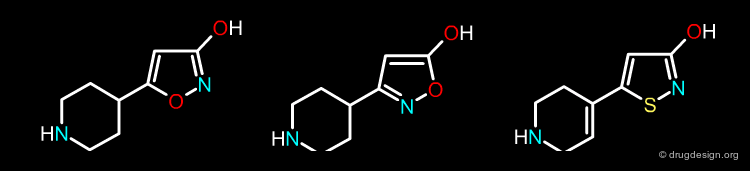
Shape¶
The following three bioisostere molecules have virtually the same shape (for clarity non-essential hydrogen atoms were omitted from the views).
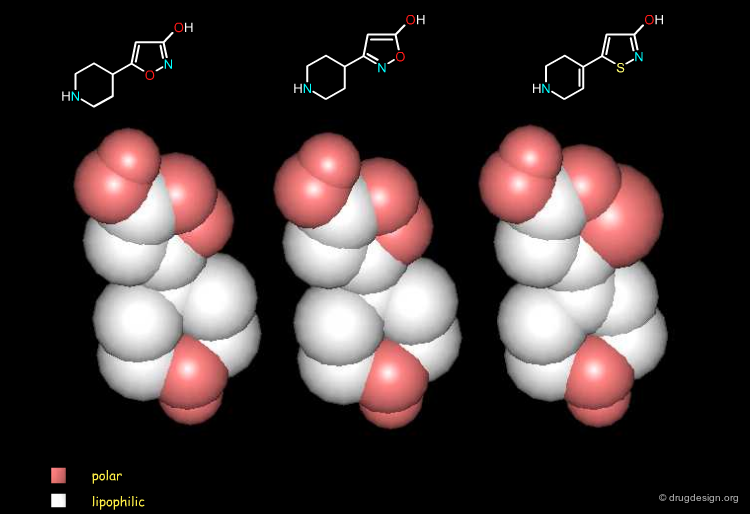
Lipophilicity¶
They also present the same distribution of lipophilic and polar groups (for clarity non-essential hydrogen atoms were omitted from the views).
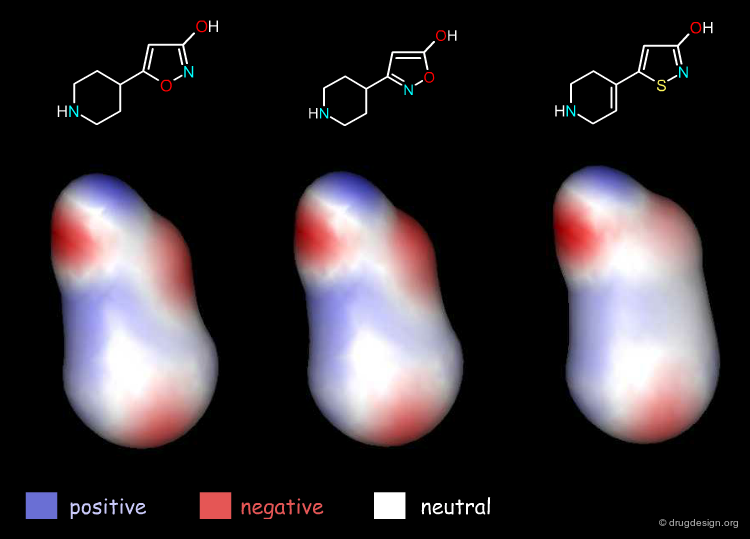
Electronic Distribution¶
If we analyze the 3D distribution of the electrostatic potential in the surroundings of the three molecules, they prove to be very similar (for clarity non-essential hydrogen atoms were omitted from the views).
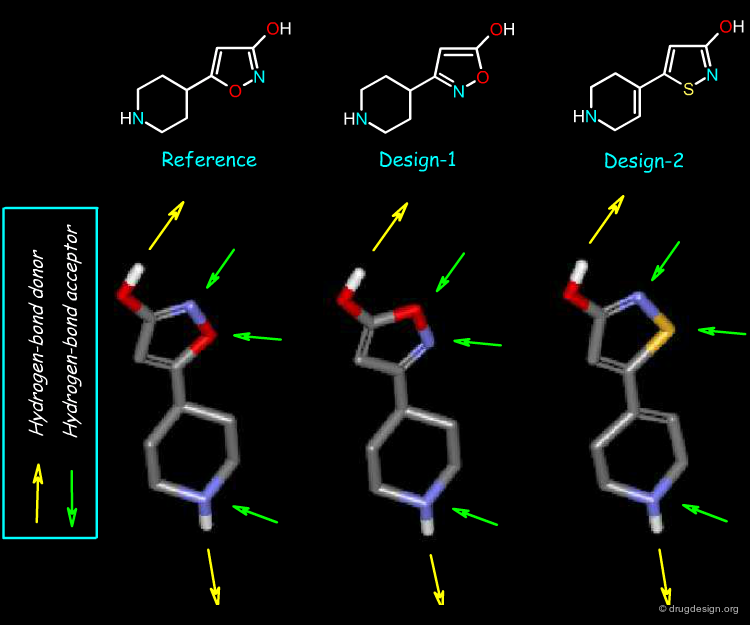
Hydrogen-Bond Capacity¶
Finally, in terms of their hydrogen-bonding capacity the three molecules have the same capabilities. These molecules share similar features from multiple points of view. It is up to the chemist to decide whether these properties are sufficient to consider them as bioisosteres, or whether other physicochemical parameters should also be considered before starting the development of a synthetic program.
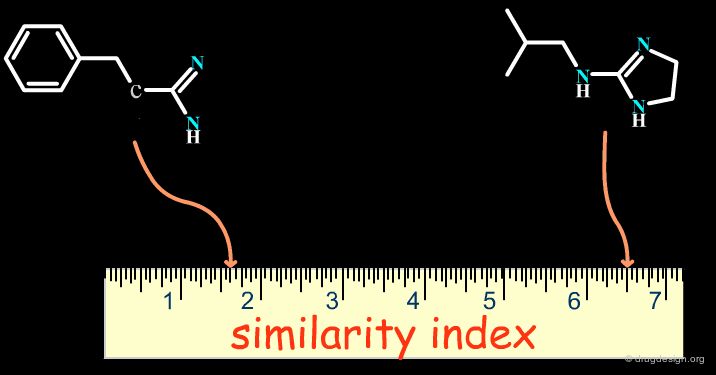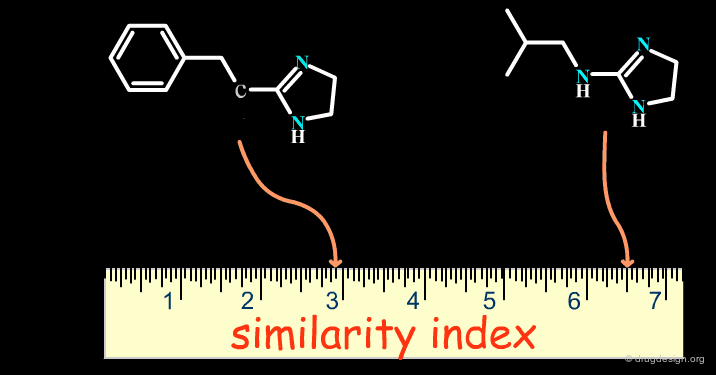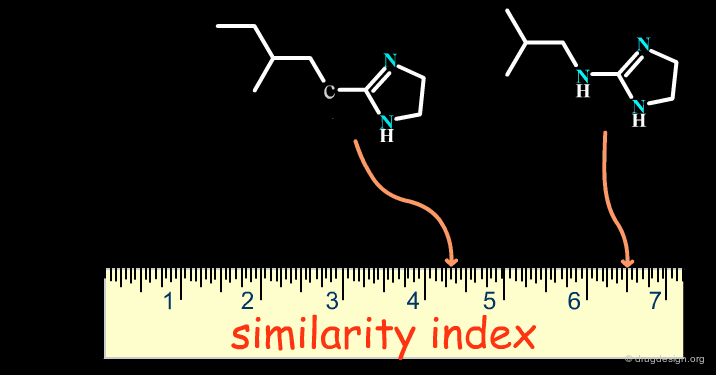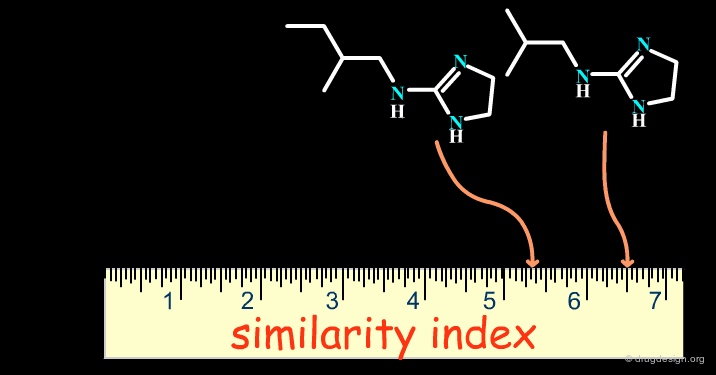
Can Bioisosterism be Quantified?¶
Successes obtained using bioisosteric replacements provided the foundation for the development of Quantitative Structure-Activity Relationships (QSAR) in drug design where candidate bioisosteric groups could be compared in terms of parameters including electronic properties (e.g. Σ), lipophilicity (e.g. π) and steric properties (e.g. MR). Recently, cheminformatics has broadened the field by assigning similarity indexes to molecular structures, as indicated on the following page.
articles
Bioisosterism: Quantitation of Structure and Property Effects Lemont B. Kier and Lowell H. Hall Chemistry and Biodiversity 1 (1) 2004
Isosterism and molecular modification in drug design Thornber C.W. Chem.Soc.Rev 8 1979
The Cheminformatics Era¶
With the advent of computers in drug design, cheminformatics has emerged as a new discipline which has given an entirely new dimension to bioisosterism. Theoretical considerations now make it possible to treat bioisosterism in a quantitative manner with more modern methods for measuring molecular similarity (see the chapter on this topic in the cheminformatics volume).
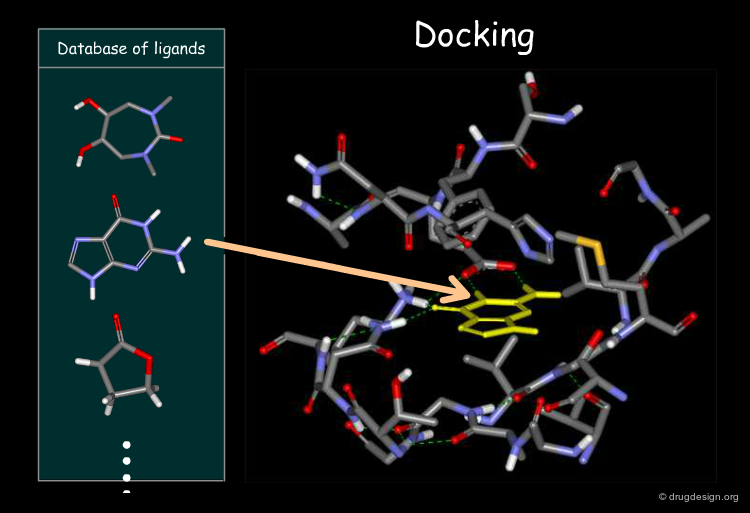
Docking can be used to Generate Bioisosteres¶
When the 3D structure of the target protein is known, docking can be used to generate isosteres. The docking procedure takes into account the van der Waals, hydrogen bonding and electrostatic forces and can be automated to search in a database of fragments. The scoring function associated with the docking treatment enables discrimination between good and bad isosteres.
Strategic and Financial Considerations¶
Research based on the development of analog compounds is attractive because of the limited risk and also because no important investment is needed in fundamental research. On the other hand, the "me-too" drug will be in competition with the other analogs on the market and the effects that will be observed in the long term are unpredictable; they can be outstanding or catastrophic. Sales of analog drugs are estimated to represent two-thirds of all the small molecules market.
articles
Similarity in drugs: reflections on analogue design Wermuth CG Drug Discov Today 11(7-8) 2006
book
IUPAC Analogue-Based Drug Discovery Wiley-VCH 2006
Examples of Success and Failures¶
Certain drugs that were introduced as a copy of an existing drug eventually became blockbusters and garnered a greater part of the market than the original 'pioneer' drug because of concrete additional advantages. Below are examples of successful drugs and others that failed for the two types of research (place the cursor on the name of each drug to visualize additional information).
Copyright © 2024 drugdesign.org